Integrity of Neuronal Size in the Entorhinal Cortex Is a Biological Substrate of Exceptional Cognitive Aging
- PMID: 36180225
- PMCID: PMC9665923
- DOI: 10.1523/JNEUROSCI.0679-22.2022
Integrity of Neuronal Size in the Entorhinal Cortex Is a Biological Substrate of Exceptional Cognitive Aging
Abstract
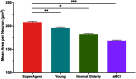
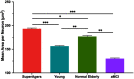
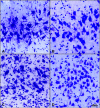
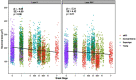
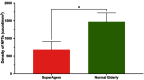
Average aging is associated with a gradual decline of memory capacity. SuperAgers are humans ≥80 years of age who show exceptional episodic memory at least as good as individuals 20-30 years their junior. This study investigated whether neuronal integrity in the entorhinal cortex (ERC), an area critical for memory and selectively vulnerable to neurofibrillary degeneration, differentiated SuperAgers from cognitively healthy younger individuals, cognitively average peers ("Normal Elderly"), and individuals with amnestic mild cognitive impairment. Postmortem sections of the ERC were stained with cresyl violet to visualize neurons and immunostained with mouse monoclonal antibody PHF-1 to visualize neurofibrillary tangles. The cross-sectional area (i.e., size) of layer II and layer III/V ERC neurons were quantified. Two-thirds of total participants were female. Unbiased stereology was used to quantitate tangles in a subgroup of SuperAgers and Normal Elderly. Linear mixed-effect models were used to determine differences across groups. Quantitative measurements found that the soma size of layer II ERC neurons in postmortem brain specimens were significantly larger in SuperAgers compared with all groups (p < 0.05)-including younger individuals 20-30 years their junior (p < 0.005). SuperAgers had significantly fewer stereologically quantified Alzheimer's disease-related neurofibrillary tangles in layer II ERC than Normal Elderly (p < 0.05). This difference in tangle burden in layer II between SuperAgers and Normal Elderly suggests that tangle-bearing neurons may be prone to shrinkage during aging. The finding that SuperAgers show ERC layer II neurons that are substantially larger even compared with individuals 20-30 years younger is remarkable, suggesting that layer II ERC integrity is a biological substrate of exceptional memory in old age.SIGNIFICANCE STATEMENT Average aging is associated with a gradual decline of memory. Previous research shows that an area critical for memory, the entorhinal cortex (ERC), is susceptible to the early formation of Alzheimer's disease neuropathology, even during average (or typical) trajectories of aging. The Northwestern University SuperAging Research Program studies unique individuals known as SuperAgers, individuals ≥80 years old who show exceptional memory that is at least as good as individuals 20-30 years their junior. In this study, we show that SuperAgers harbor larger, healthier neurons in the ERC compared with their cognitively average same-aged peers, those with amnestic mild cognitive impairment, and - remarkably - even compared with individuals 20-30 years younger. We conclude that larger ERC neurons are a biological signature of the SuperAging trajectory.
Keywords: Alzheimer's disease; SuperAging; entorhinal cortex; neurofibrillary tangles; neuronal integrity.
Copyright © 2022 the authors.
Figures


References
-
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:270–279. 10.1016/j.jalz.2011.03.008 - DOI - PMC - PubMed
-
- Braak H, Braak E (1991a) Morphological changes in the human cerebral cortex in dementia. J Hirnforsch 32:277–282. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
