The Longer-Term Benefits and Harms of Glucagon-Like Peptide-1 Receptor Agonists: a Systematic Review and Meta-Analysis
- PMID: 34508290
- PMCID: PMC8810987
- DOI: 10.1007/s11606-021-07105-9
The Longer-Term Benefits and Harms of Glucagon-Like Peptide-1 Receptor Agonists: a Systematic Review and Meta-Analysis
Abstract
Background: Previous meta-analyses of the benefits and harms of glucagon-like peptide-1 receptor agonists (GLP1RAs) have been limited to specific outcomes and comparisons and often included short-term results. We aimed to estimate the longer-term effects of GLP1RAs on cardiovascular risk factors, microvascular and macrovascular complications, mortality, and adverse events in patients with type 2 diabetes, compared to placebo and other anti-hyperglycemic medications.
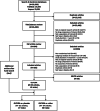
Methods: We searched PubMed, Scopus, and clinicaltrials.gov (inception-July 2019) for randomized controlled trials ≥ 52 weeks' duration that compared a GLP1RA to placebo or other anti-hyperglycemic medication and included at least one outcome of interest. Outcomes included cardiovascular risk factors, microvascular and macrovascular complications, all-cause mortality, and treatment-related adverse events. We performed random effects meta-analyses to give summary estimates using weighted mean differences (MD) and pooled relative risks (RR). Risk of bias was assessed using the Cochrane Collaboration risk of bias in randomized trials tool. Quality of evidence was summarized using the Grading of Recommendations, Assessment, Development, and Evaluation approach. The study was registered a priori with PROSPERO (CRD42018090506).
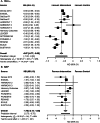
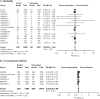
Results: Forty-five trials with a mean duration of 1.7 years comprising 71,517 patients were included. Compared to placebo, GLP1RAs reduced cardiovascular risk factors, microvascular complications (including renal events, RR 0.85, 0.80-0.90), macrovascular complications (including stroke, RR 0.86, 0.78-0.95), and mortality (RR 0.89, 0.84-0.94). Compared to other anti-hyperglycemic medications, GLP1RAs only reduced cardiovascular risk factors. Increased gastrointestinal events causing treatment discontinuation were observed in both comparisons.
Discussion: GLP1RAs reduced cardiovascular risk factors and increased gastrointestinal events compared to placebo and other anti-hyperglycemic medications. GLP1RAs also reduced MACE, stroke, renal events, and mortality in comparisons with placebo; however, analyses were inconclusive for comparisons with other anti-hyperglycemic medications. Given the high costs of GLP1RAs, the lack of long-term evidence comparing GLP1RAs to other anti-hyperglycemic medications has significant policy and clinical practice implications.
Keywords: diabetes; glucagon-like peptide-1 receptor agonists; meta-analysis; systematic review.
© 2021. Society of General Internal Medicine.
Conflict of interest statement
The authors declare that they do not have a conflict of interest.
Figures
















References
-
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Research and Clinical Practice. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. - DOI - PubMed
-
- Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical Update: Cardiovascular Disease in Diabetes Mellitus: Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes Mellitus - Mechanisms, Management, and Clinical Considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. - DOI - PMC - PubMed
-
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England). 2019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

