Exenatide Attenuates Obesity-Induced Mitochondrial Dysfunction by Activating SIRT1 in Renal Tubular Cells
- PMID: 34434166
- PMCID: PMC8380782
- DOI: 10.3389/fendo.2021.622737
Exenatide Attenuates Obesity-Induced Mitochondrial Dysfunction by Activating SIRT1 in Renal Tubular Cells
Abstract
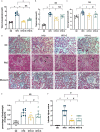
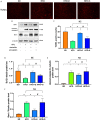
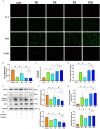
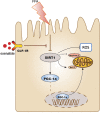
Saturated free fatty acid (FFA)-induced lipotoxicity plays an important role in obesity-induced kidney injury. Exenatide, a Glucagon-like peptide-1 receptor agonist(GLP-1RA), protects against high-fat diet (HFD)-induced kidney injury. The precise mechanism needs to be further explored. This study investigated whether exenatide protects against FFA-induced tubular epithelial cells (TECs) lipotoxicity and elucidated its underlying mechanisms. Here, we show that exenatide treatment reversed HFD induced TECs injuries, including TECs apoptosis and SIRT1 downregulation. The efficacy of exenatide was better than simvastatin. In palmitate (PA)-stimulated HK2 cells, exenatide treatment reversed the downregulation of SIRT1 and prevented an increase in reactive oxygen species (ROS) production, a decrease in mitochondrial membrane potential, and mitochondrial apoptosis. The renal-protective effects of exenatide on the generation of mitochondrial ROS and mitochondrial apoptosis were blocked by inhibiting SIRT1 activation. Collectively, these findings show that exenatide was superior to simvastatin in the treatment of obesity-TECs injuries, the mechanism is partially through SIRT1 restoration, which directly reverses mitochondrial dysfunction and apoptosis.
Keywords: SIRT1; glucagon-like peptide-1 receptor agonist; mitochondrial dysfunction; obesity; renal tubular cells.
Copyright © 2021 Wang, He, Wei, Mei, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

