Safety and nonclinical and clinical pharmacokinetics of PC945, a novel inhaled triazole antifungal agent
- PMID: 33340279
- PMCID: PMC7749516
- DOI: 10.1002/prp2.690
Safety and nonclinical and clinical pharmacokinetics of PC945, a novel inhaled triazole antifungal agent
Abstract
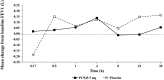
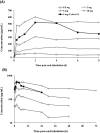
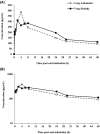
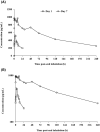
PC945 is a novel antifungal triazole formulated for nebulized delivery to treat lung Aspergillus infections. Pharmacokinetic and safety profiles from nonclinical studies and clinical trials in healthy subjects, and subjects with mild asthma were characterized. Toxicokinetics were assessed following daily 2-hour inhalation for 14 days. Potential for drug-drug interactions was evaluated using pooled human liver microsomes. Clinical safety and pharmacokinetics were assessed following (a) single inhaled doses (0.5-10 mg), (b) 7-day repeat doses (5 mg daily) in healthy subjects; (c) a single dose (5 mg) in subjects with mild asthma. Cmax occurred 4 hours (rats) or immediately (dogs) after a single dose. PC945 lung concentrations were substantially higher (>2000-fold) than those in plasma. PC945 only inhibited CYP3A4/5 substrate metabolism (IC50 : 1.33 µM [testosterone] and 0.085 µM [midazolam]). Geometric mean Cmax was 322 pg/mL (healthy subjects) and 335 pg/mL (subjects with mild asthma) 4-5 hours (median tmax ) after a single inhalation (5 mg). Following repeat, once daily inhalation (5 mg), Day 7 Cmax was 951 pg/mL (0.0016 µM) 45 minutes after dosing. Increases in Cmax and AUC0-24h were approximately dose-proportional (0.5-10 mg). PC945 administration was well tolerated in both healthy subjects and subjects with mild asthma. Treatment-emergent adverse events were mild/moderate and resolved before the study ended. No clinically significant lung function changes were observed. PC945 pharmacokinetics translated from nonclinical species to humans showed slow absorption from lungs and low systemic exposure, thereby limiting the potential for adverse side effects and drug interactions commonly seen with systemically delivered azoles.
Keywords: PC945; antifungal; drug-drug interaction; first-in-human; inhaled administration; pharmacokinetics; safety.
© 2020 The Authors. Pharmacology Research & Perspectives published by John Wiley & Sons Ltd, British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics.
Conflict of interest statement
All authors have completed the Unified Competing Interest form at
Figures
References
-
- Orlowski HLP, McWilliams S, Mellnick VM, et al. Imaging spectrum of invasive fungal and fungal‐like infections. Radiographics. 2017;37(4):1119‐1134. - PubMed
-
- Perlin DS, Rautemaa‐Richardson R, Alastruey‐Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383‐e392. - PubMed
-
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270‐277. - PubMed
-
- Limper AH, Knox KS, Sarosi GA, et al. An Official American Thoracic Society Statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183(1):96‐128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

