Structural insights into differences in G protein activation by family A and family B GPCRs
- PMID: 32732395
- PMCID: PMC7954662
- DOI: 10.1126/science.aba3373
Structural insights into differences in G protein activation by family A and family B GPCRs
Abstract
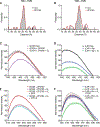
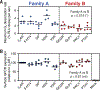
Family B heterotrimeric guanine nucleotide-binding protein (G protein)-coupled receptors (GPCRs) play important roles in carbohydrate metabolism. Recent structures of family B GPCR-Gs protein complexes reveal a disruption in the α-helix of transmembrane segment 6 (TM6) not observed in family A GPCRs. To investigate the functional impact of this structural difference, we compared the structure and function of the glucagon receptor (GCGR; family B) with the β2 adrenergic receptor (β2AR; family A). We determined the structure of the GCGR-Gs complex by means of cryo-electron microscopy at 3.1-angstrom resolution. This structure shows the distinct break in TM6. Guanosine triphosphate (GTP) turnover, guanosine diphosphate release, GTP binding, and G protein dissociation studies revealed much slower rates for G protein activation by the GCGR compared with the β2AR. Fluorescence and double electron-electron resonance studies suggest that this difference is due to the inability of agonist alone to induce a detectable outward movement of the cytoplasmic end of TM6.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures
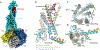


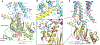
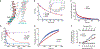
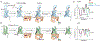
Comment in
-
Fine-tuning receptor-G protein activation and signaling.Science. 2020 Jul 31;369(6503):507-508. doi: 10.1126/science.abc9291. Science. 2020. PMID: 32732411 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

