Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage
- PMID: 32574561
- PMCID: PMC7342013
- DOI: 10.1016/j.cmet.2020.06.004
Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage
Abstract
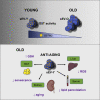
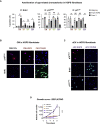
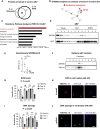
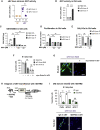
Aging is a process of cellular and tissue dysfunction characterized by different hallmarks, including cellular senescence. However, there is proof that certain features of aging and senescence can be ameliorated. Here, we provide evidence that small extracellular vesicles (sEVs) isolated from primary fibroblasts of young human donors ameliorate certain biomarkers of senescence in cells derived from old and Hutchinson-Gilford progeria syndrome donors. Importantly, sEVs from young cells ameliorate senescence in a variety of tissues in old mice. Mechanistically, we identified sEVs to have intrinsic glutathione-S-transferase activity partially due to the high levels of expression of the glutathione-related protein (GSTM2). Transfection of recombinant GSTM2 into sEVs derived from old fibroblasts restores their antioxidant capacity. sEVs increase the levels of reduced glutathione and decrease oxidative stress and lipid peroxidation both in vivo and in vitro. Altogether, our data provide an indication of the potential of sEVs as regenerative therapy in aging.
Keywords: 4-HNE; EV; GSH; GST; ROS; SASP; aging; extracellular vesicles; glutathione metabolism; glutathione-S-transferase; lipid peroxidation; rejuvenation; senescence; senescence-associated secretory phenotype.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no conflicts of interest.
Figures



Comment in
-
Small extracellular vesicles combat senescence.Nat Rev Mol Cell Biol. 2020 Sep;21(9):498-499. doi: 10.1038/s41580-020-0271-7. Nat Rev Mol Cell Biol. 2020. PMID: 32661343 No abstract available.
References
-
- Acosta J.C., O’Loghlen A., Banito A., Guijarro M.V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

