Evaluation of the Performance of the IMMY sona Aspergillus Galactomannan Lateral Flow Assay When Testing Serum To Aid in Diagnosis of Invasive Aspergillosis
- PMID: 32188687
- PMCID: PMC7269407
- DOI: 10.1128/JCM.00053-20
Evaluation of the Performance of the IMMY sona Aspergillus Galactomannan Lateral Flow Assay When Testing Serum To Aid in Diagnosis of Invasive Aspergillosis
Abstract
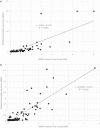
Management of invasive aspergillosis has been improved by biomarker assays, but limited accessibility and batch testing limit the impact. Lateral flow assays (LFA) are a simple method for use outside specialist centers, provided performance is acceptable. The objective of this study was to determine the performance of the recently released IMMY sona Aspergillus LFA when testing serum samples. The study took the form of a retrospective, anonymous case/control study comprising 179 serum samples from 136 patients with invasive fungal disease, previously documented using recently revised internationally accepted definitions. The LFA was performed following the manufacturer's instructions using a cube reader to generate a galactomannan index (GMI). Performance parameters were determined, and receiver operator characteristic (ROC) analysis was used to identify an optimal threshold. Concordance with the Bio-Rad Aspergillus Ag assay (GM-EIA) was performed. At the recommended positivity threshold (GMI ≥ 0.5), LFA sensitivity and specificity were 96.9% (31/32) and 98% (98/100), respectively. ROC analysis confirmed the optimal threshold and generated an area under the curve of 0.9919. Qualitative agreement between LFA and GM-EIA was 89.0%, generating a Kappa statistic of 0.698, representing good agreement, with most discordance arising due to false-negative GM-EIA samples that were positive by LFA. The median GMI generated by the LFA was significantly greater than that generated by the GM-EIA. The IMMY sona Aspergillus LFA, when used with a cube reader, provides a rapid alternative to the well-established GM-EIA, potentially detecting more GM epitopes and enhancing sensitivity.
Keywords: Aspergillus diagnostics; galactomannan; invasive aspergillosis; lateral flow assay; serum.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, Szer J, Halliday CL, Gilroy NM, Moore J, Schwarer AP, Guy S, Bajel A, Tramontana AR, Spelman T, Slavin MA, Australasian Leukaemia Lymphoma Group and the Australia and New Zealand Mycology Interest Group. 2013. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis 13:519–528. doi:10.1016/S1473-3099(13)70076-8. - DOI - PubMed
-
- Aguado JM, Vázquez L, Fernández-Ruiz M, Villaescusa T, Ruiz-Camps I, Barba P, Silva JT, Batlle M, Solano C, Gallardo D, Heras I, Polo M, Varela R, Vallejo C, Olave T, López-Jiménez J, Rovira M, Parody R, Cuenca-Estrella M, PCRAGA Study Group, Spanish Stem Cell Transplantation Group, Study Group of Medical Mycology of the Spanish Society of Clinical Microbiology and Infectious Diseases, Spanish Network for Research in Infectious Diseases. 2015. Serum galactomannan versus a combination of galactomannan and polymerase chain reaction-based Aspergillus DNA detection for early therapy of invasive aspergillosis in high-risk hematological patients: a randomized controlled trial. Clin Infect Dis 60:405–414. doi:10.1093/cid/ciu833. - DOI - PubMed
-
- Leeflang MM, Debets-Ossenkopp YJ, Visser CE, Scholten RJ, Hooft L, Bijlmer HA, Reitsma JB, Bossuyt PM, Vandenbroucke-Grauls CM. 2009. Galactomannan detection for invasive aspergillosis in immunocompromized patients. Cochrane Database Syst Rev 8:CD007394. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

