Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens
- PMID: 31984598
- PMCID: PMC7318331
- DOI: 10.1111/dom.13979
Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens
Abstract
Aim: To assess the efficacy and tolerability of tirzepatide treatment using three different dose-escalation regimens in patients with type 2 diabetes.
Materials and methods: In this double-blind, placebo-controlled study, patients were randomized (1:1:1:1) to receive either once-weekly subcutaneous tirzepatide or placebo. The tirzepatide dose groups and dose-escalation regimens were: 12 mg (4 mg weeks 0-3; 8 mg weeks 4-7; 12 mg weeks 8-11), 15 mg-1 (2.5 mg weeks 0-1; 5 mg weeks 2-3; 10 mg weeks 4-7; 15 mg weeks 8-11) and 15 mg-2 (2.5 mg weeks 0-3; 7.5 mg weeks 4-7; 15 mg weeks 8-11). The primary objective was to compare tirzepatide with placebo in HbA1c change from baseline at 12 weeks.
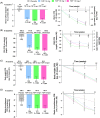
Results: Overall, 111 patients were randomized: placebo, 26; tirzepatide 12 mg, 29; tirzepatide 15 mg-1, 28; tirzepatide 15 mg-2, 28. The mean age was 57.4 years, HbA1c 8.4% and body mass index 31.9 kg/m2 . At week 12, absolute HbA1c change from baseline (SE) was greater in the tirzepatide treatment groups compared with placebo (placebo, +0.2% [0.21]; 12 mg, -1.7% [0.19]; 15 mg-1, -2.0% [0.20]; 15 mg-2, -1.8% [0.19]). The incidence of nausea was: placebo, 7.7%; 12 mg group, 24.1%; 15 mg-1 group, 39.3%; 15 mg-2 group, 35.7%. Three patients discontinued the treatment because of adverse events, one from each of the placebo, 12 mg and 15 mg-1 groups.
Conclusions: Tirzepatide treatment for 12 weeks resulted in clinically significant reductions in HbA1c. This suggests that lower starting doses and smaller dose increments are associated with a more favourable side effect profile.
Keywords: antidiabetic drug; glucagon-like peptide-1 analogue; glucose-dependent insulinotropic peptide; incretin therapy; randomized trial; type 2 diabetes.
© 2020 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
J.P.F. declares grants from Eli Lilly during the conduct of the study; grants from AbbVie, Akcea, Allergan, AstraZeneca, Boehringer Ingelheim, BMS, Cirius, CymaBay, Enanta, Genentech, Intercept, Janssen, Johnson and Johnson, Lexicon, Ligand, Madrigal, Merck, Mylan, NGM, Novartis, Novo Nordisk, Pfizer, Sanofi and Theracos; advisory board and consulting from Boehringer Ingelheim, Gilead, Johnson and Johnson, Eli Lilly, Merck, Novo Nordisk and Sanofi; and speaker bureau from Merck, Sanofi. M.A.N. has received compensation for lectures or advisory board membership from AstraZeneca, Berlin‐Chemie/Menarini, Medscape, Merck Sharp & Dohme, Novo Nordisk, Sanofi‐Aventis, Takeda and Sun Pharma; and received grant support from Berlin‐Chemie/Menarini, Eli Lilly and Company, Merck Sharp & Dohme and Novo Nordisk. J.V. declares research support from Aptinyx, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Genentech, Lexicon, Melior, Mylan, Novo Nordisk, Regenesis and Sanofi. R.B., X.C., C.B., S.U., Z.M., D.A.R. and A.H. are employees and shareholders of Eli Lilly and Company.
Figures

References
-
- Drucker DJ, Nauck MA. The incretin system: Glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696‐1705. - PubMed
-
- Nauck MA, Meier JJ. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016;4:525‐536. - PubMed
-
- Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev. 2007;87:1409‐1439. - PubMed
-
- Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19:336‐347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

