Epidemiology of a bubonic plague outbreak in Glasgow, Scotland in 1900
- PMID: 30800398
- PMCID: PMC6366177
- DOI: 10.1098/rsos.181695
Epidemiology of a bubonic plague outbreak in Glasgow, Scotland in 1900
Abstract
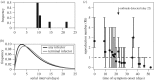
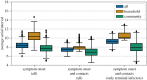
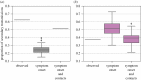
On 3 August 1900, bubonic plague (Yersinia pestis) broke out in Glasgow for the first time during the Third Pandemic. The local sanitary authorities rigorously tracked the spread of the disease and they found that nearly all of the 35 cases could be linked by contact with a previous case. Despite trapping hundreds of rats in the area, there was no evidence of a rat epizootic and the investigators speculated that the outbreak could be due to human-to-human transmission of bubonic plague. Here we use a likelihood-based method to reconstruct transmission trees for the outbreak. From the description of the outbreak and the reconstructed trees, we infer several epidemiological parameters. We found that the estimated mean serial interval was 7.4-9.2 days and the mean effective reproduction number dropped below 1 after implementation of control measures. We also found a high rate of secondary transmissions within households and observations of transmissions from individuals who were not terminally septicaemic. Our results provide important insights into the epidemiology of a bubonic plague outbreak during the Third Pandemic in Europe.
Keywords: Third Pandemic; Yersinia pestis; human ectoparasite transmission; reproduction number; serial interval.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Dennis DT, Gage KL, Gratz NG, Poland JD, Tikhomirov E. 1999. Plague manual: epidemiology, distribution, surveillance and control, pp. 1–72. Geneva, Switzerland: World Health Organization.
-
- Migliani R, Chanteau S, Rahalison L, Ratsitorahina M, Boutin JP, Ratsifasoamanana L, Roux J. 2006. Epidemiological trends for human plague in Madagascar during the second half of the 20th century: a survey of 20,900 notified cases. Trop. Med. Int. Health 11, 1228–1237. (10.1111/j.1365-3156.2006.01677.x) - DOI - PubMed
LinkOut - more resources
Full Text Sources

