SIRT1 Attenuates Kidney Disorders in Male Offspring Due to Maternal High-Fat Diet
- PMID: 30641941
- PMCID: PMC6356703
- DOI: 10.3390/nu11010146
SIRT1 Attenuates Kidney Disorders in Male Offspring Due to Maternal High-Fat Diet
Abstract
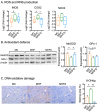
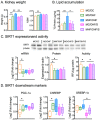
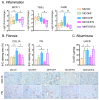
Maternal obesity has been associated with kidney disorders in male offspring. Our previous studies have demonstrated that Sirtuin (SIRT)1, an essential regulator of metabolic stress responses, is suppressed in the offspring as the result of maternal high-fat diet (HFD) consumption, which is likely to underpin the adverse metabolic and renal outcomes. To examine if SIRT1 overexpression or activation early in life can protect the offspring kidney, wild-type (WT) and transgenic (Tg) offspring were born to the same diet-induced obese female C57BL/6 mice through breeding with hemizygous SIRT1-transgenic (Tg) male mice and examined for renal pathological changes. In separate experiments, SIRT1 activator SRT1720 (25 mg/kg/2 days i.p) was administrated in WT offspring over 6 weeks of postnatal high-fat diet exposure. The results show that offspring born to obese dams have increased kidney weight, higher levels of renal triglycerides, and increased expression of oxidative stress, inflammatory, and fibrotic markers, as well as increased albuminuria compared to offspring of control dams. Both SIRT1 overexpression and SRT1720 treatment attenuated renal lipid contents and expression of lipogenesis, oxidative stress, and inflammatory markers; however, fibrosis was modestly reduced and albuminuria was not affected. The findings suggest that SIRT1 therapy can ameliorate some pathological mechanisms of kidney programming due to maternal obesity but may not be sufficient to prevent the resulting chronic kidney injury.
Keywords: Obesity; chronic kidney disease; foetal programming; sirtuin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Oben J.A., Mouralidarane A., Samuelsson A.-M., Matthews P.J., Morgan M.L., Mckee C., Soeda J., Fernandez-Twinn D.S., Martin-Gronert M.S., Ozanne S.E. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J. Hepatol. 2010;52:913–920. doi: 10.1016/j.jhep.2009.12.042. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

