Hog1 Regulates Stress Tolerance and Virulence in the Emerging Fungal Pathogen Candida auris
- PMID: 30355673
- PMCID: PMC6200985
- DOI: 10.1128/mSphere.00506-18
Hog1 Regulates Stress Tolerance and Virulence in the Emerging Fungal Pathogen Candida auris
Abstract
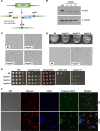
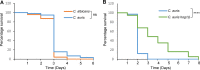
Candida auris has recently emerged as an important, multidrug-resistant fungal pathogen of humans. Comparative studies indicate that despite high levels of genetic divergence, C. auris is as virulent as the most pathogenic member of the genus, Candida albicans However, key virulence attributes of C. albicans, such as morphogenetic switching, are not utilized by C. auris, indicating that this emerging pathogen employs alternative strategies to infect and colonize the host. An important trait required for the pathogenicity of many fungal pathogens is the ability to adapt to host-imposed stresses encountered during infection. Here, we investigated the relative resistance of C. auris and other pathogenic Candida species to physiologically relevant stresses and explored the role of the evolutionarily conserved Hog1 stress-activated protein kinase (SAPK) in promoting stress resistance and virulence. In comparison to C. albicans, C. auris is relatively resistant to hydrogen peroxide, cationic stress, and cell-wall-damaging agents. However, in contrast to other Candida species examined, C. auris was unable to grow in an anaerobic environment and was acutely sensitive to organic oxidative-stress-inducing agents. An analysis of C. aurishog1Δ cells revealed multiple roles for this SAPK in stress resistance, cell morphology, aggregation, and virulence. These data demonstrate that C. auris has a unique stress resistance profile compared to those of other pathogenic Candida species and that the Hog1 SAPK has pleiotropic roles that promote the virulence of this emerging pathogen.IMPORTANCE The rapid global emergence and resistance of Candidaauris to current antifungal drugs highlight the importance of understanding the virulence traits exploited by this human fungal pathogen to cause disease. Here, we characterize the stress resistance profile of C. auris and the role of the Hog1 stress-activated protein kinase (SAPK) in stress resistance and virulence. Our findings that C. auris is acutely sensitive to certain stresses may facilitate control measures to prevent persistent colonization in hospital settings. Furthermore, our observation that the Hog1 SAPK promotes C. auris virulence akin to that reported for many other pathogenic fungi indicates that antifungals targeting Hog1 signaling would be broad acting and effective, even on emerging drug-resistant pathogens.
Keywords: Candida auris; pathogenesis; stress adaptation; stress kinases.
Copyright © 2018 Day et al.
Figures



References
-
- Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. 2016. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 5:35. doi:10.1186/s13756-016-0132-5. - DOI - PMC - PubMed
-
- Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2017. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus-United States, May 2013-August 2016. Am J Transplant 17:296–299. doi:10.1111/ajt.14121. - DOI - PubMed
-
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi:10.1093/cid/ciw691. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/K016393/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 097377/WT_/Wellcome Trust/United Kingdom
- 097377/101873/WT_/Wellcome Trust/United Kingdom
- BB/P020119/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 101873/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
