Lixisenatide Reduces Chylomicron Triacylglycerol by Increased Clearance
- PMID: 30215735
- PMCID: PMC6300412
- DOI: 10.1210/jc.2018-01176
Lixisenatide Reduces Chylomicron Triacylglycerol by Increased Clearance
Abstract
Context: Glucagon-like peptide-1 (GLP-1) agonists control postprandial glucose and lipid excursion in type 2 diabetes; however, the mechanisms are unclear.
Objective: To determine the mechanisms of postprandial lipid and glucose control with lixisenatide (GLP-1 analog) in type 2 diabetes.
Design: Randomized, double-blind, cross-over study.
Setting: Centre for Diabetes, Endocrinology, and Research, Royal Surrey County Hospital, Guildford, United Kingdom.
Patients: Eight obese men with type 2 diabetes [age, 57.3 ± 1.9 years; body mass index, 30.3 ± 1.0 kg/m2; glycosylated hemoglobin, 66.5 ± 2.6 mmol/mol (8.2% ± 0.3%)].
Interventions: Two metabolic studies, 4 weeks after lixisenatide or placebo, with cross-over and repetition of studies.
Main outcome measures: Study one: very-low-density lipoprotein (VLDL) and chylomicron (CM) triacylglycerol (TAG) kinetics were measured with an IV bolus of [2H5]glycerol in a 12-hour study, with hourly feeding. Oral [13C]triolein, in a single meal, labeled enterally derived TAG. Study two: glucose kinetics were measured with [U-13C]glucose in a mixed-meal (plus acetaminophen to measure gastric emptying) and variable IV [6,6-2H2]glucose infusion.
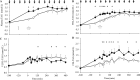
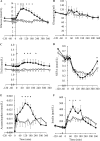
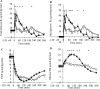
Results: Study one: CM-TAG (but not VLDL-TAG) pool-size was lower with lixisenatide (P = 0.046). Lixisenatide reduced CM [13C]oleate area under the curve (AUC)60-480min concentration (P = 0.048) and increased CM-TAG clearance, with no effect on CM-TAG production rate. Study two: postprandial glucose and insulin AUC0-240min were reduced with lixisenatide (P = 0.0051; P < 0.05). Total glucose production (P = 0.015), rate of glucose appearance from the meal (P = 0.0098), and acetaminophen AUC0-360min (P = 0.006) were lower with lixisenatide than with placebo.
Conclusions: Lixisenatide reduced [13C]oleate concentrations, derived from a single meal in CM-TAG and glucose rate of appearance from the meal through delayed gastric emptying. However, day-long CM production, measured with repeated meal feeding, was not reduced by lixisenatide and decreased CM-TAG concentration resulted from increased CM-TAG clearance.
Trial registration: ClinicalTrials.gov NCT02049034.
Figures

References
-
- Patsch JR, Miesenböck G, Hopferwieser T, Mühlberger V, Knapp E, Dunn JK, Gotto AM Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12(11):1336–1345. - PubMed
-
- Sharrett AR, Chambless LE, Heiss G, Paton CC, Patsch W. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women. The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 1995;15(12):2122–2129. - PubMed
-
- Eberly LE, Stamler J, Neaton JD; Multiple Risk Factor Intervention Trial Research Group . Relation of triglyceride levels, fasting and nonfasting, to fatal and nonfatal coronary heart disease. Arch Intern Med. 2003;163(9):1077–1083. - PubMed
-
- Schrezenmeir J, Keppler I, Fenselau S, Weber P, Biesalski HK, Probst R, Laue C, Zuchhold HD, Prellwitz W, Beyer J. The phenomenon of a high triglyceride response to an oral lipid load in healthy subjects and its link to the metabolic syndrome. Ann N Y Acad Sci. 1993;683:302–314. - PubMed
-
- Meier JJ, Gethmann A, Götze O, Gallwitz B, Holst JJ, Schmidt WE, Nauck MA. Glucagon-like peptide 1 abolishes the postprandial rise in triglyceride concentrations and lowers levels of non-esterified fatty acids in humans. Diabetologia. 2006;49(3):452–458. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

