Fungal Resistance to Echinocandins and the MDR Phenomenon in Candida glabrata
- PMID: 30200517
- PMCID: PMC6162769
- DOI: 10.3390/jof4030105
Fungal Resistance to Echinocandins and the MDR Phenomenon in Candida glabrata
Abstract
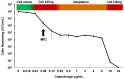
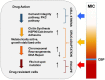
Candida glabrata has thoroughly adapted to successfully colonize human mucosal membranes and survive in vivo pressures. prior to and during antifungal treatment. Out of all the medically relevant Candida species, C. glabrata has emerged as a leading cause of azole, echinocandin, and multidrug (MDR: azole + echinocandin) adaptive resistance. Neither mechanism of resistance is intrinsic to C. glabrata, since stable genetic resistance depends on mutation of drug target genes, FKS1 and FKS2 (echinocandin resistance), and a transcription factor, PDR1, which controls expression of major drug transporters, such as CDR1 (azole resistance). However, another hallmark of C. glabrata is the ability to withstand drug pressure both in vitro and in vivo prior to stable "genetic escape". Additionally, these resistance events can arise within individual patients, which underscores the importance of understanding how this fungus is adapting to its environment and to drug exposure in vivo. Here, we explore the evolution of echinocandin resistance as a multistep model that includes general cell stress, drug adaptation (tolerance), and genetic escape. The extensive genetic diversity reported in C. glabrata is highlighted.
Keywords: Candida glabrata; FKS; MSH2; azole; drug resistance; echinocandin; tolerance.
Conflict of interest statement
D.S.P. receives funding from US National Institutes of Health and contracts from the CDC, Astellas, Scynexis, Cidara and Amplyx. He serves on advisory boards for Astellas, Cidara, Amplyx, Scynexis, and Matinas. In addition, D.S.P. has an issued US patent concerning echinocandin resistance. K.R.H. declares no competing financial interests. The authors alone are responsible for the content and writing of the paper.
Figures
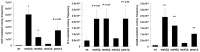
References
-
- Castanheira M., Deshpande L.M., Davis A.P., Rhomberg P.R., Pfaller M.A. Monitoring antifungal resistance in a global collection of invasive yeasts and molds: Application of clsi epidemiological cutoff values and whole-genome sequencing analysis for detection of azole resistance in Candida albicans. Antimicrob. Agents Chemother. 2017;61:e00906-17. doi: 10.1128/AAC.00906-17. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

