Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia
- PMID: 29941885
- PMCID: PMC6018213
- DOI: 10.1038/s41467-018-04926-x
Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia
Abstract
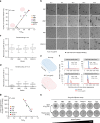
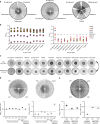
Tolerance to antifungal drug concentrations above the minimal inhibitory concentration (MIC) is rarely quantified, and current clinical recommendations suggest it should be ignored. Here, we quantify antifungal tolerance in Candida albicans isolates as the fraction of growth above the MIC, and find that it is distinct from susceptibility/resistance. Instead, tolerance is due to the slow growth of subpopulations of cells that overcome drug stress more efficiently than the rest of the population, and correlates inversely with intracellular drug accumulation. Many adjuvant drugs used in combination with fluconazole, a widely used fungistatic drug, reduce tolerance without affecting resistance. Accordingly, in an invertebrate infection model, adjuvant combination therapy is more effective than fluconazole in treating infections with highly tolerant isolates and does not affect infections with low tolerance isolates. Furthermore, isolates recovered from immunocompetent patients with persistent candidemia display higher tolerance than isolates readily cleared by fluconazole. Thus, tolerance correlates with, and may help predict, patient responses to fluconazole therapy.
Conflict of interest statement
A.L.C. has received educational grants from Pfizer, Gilead Sciences, United Medical (Brazil), MSD, and TEVAS, and a research grant from Astellas and Pfizer. The remaining authors declare no competing interests.
Figures




Comment in
-
Candida tolerates and persists.Nat Rev Microbiol. 2018 Sep;16(9):520-521. doi: 10.1038/s41579-018-0056-6. Nat Rev Microbiol. 2018. PMID: 29976950 No abstract available.
References
-
- Doern GV, Brecher SM. The clinical predictive value (or lack thereof) of the results of in vitro antimicrobial susceptibility tests. J. Clin. Microbiol. 2011;49:S11–S14. doi: 10.1128/JCM.00580-11. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

