Sirtuins in Renal Health and Disease
- PMID: 29712732
- PMCID: PMC6050939
- DOI: 10.1681/ASN.2017111218
Sirtuins in Renal Health and Disease
Abstract
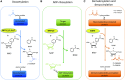
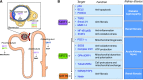
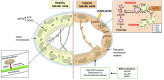
Sirtuins belong to an evolutionarily conserved family of NAD+-dependent deacetylases that share multiple cellular functions related to proliferation, DNA repair, mitochondrial energy homeostasis, and antioxidant activity. Mammalians express seven sirtuins (SIRT1-7) that are localized in different subcellular compartments. Changes in sirtuin expression are critical in several diseases, including metabolic syndrome, diabetes, cancer, and aging. In the kidney, the most widely studied sirtuin is SIRT1, which exerts cytoprotective effects by inhibiting cell apoptosis, inflammation, and fibrosis together with SIRT3, a crucial metabolic sensor that regulates ATP generation and mitochondrial adaptive response to stress. Here, we provide an overview of the biologic effects of sirtuins and the molecular targets thereof regulating renal physiology. This review also details progress made in understanding the effect of sirtuins in the pathophysiology of chronic and acute kidney diseases, highlighting the key role of SIRT1, SIRT3, and now SIRT6 as potential therapeutic targets. In this context, the current pharmacologic approaches to enhancing the activity of SIRT1 and SIRT3 will be discussed.
Keywords: Sirtuins; acute renal failure; chronic kidney disease; metabolism; mitochondria; sirtuin activators.
Copyright © 2018 by the American Society of Nephrology.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

