The double-edged sword of endoplasmic reticulum stress in uremic sarcopenia through myogenesis perturbation
- PMID: 29380555
- PMCID: PMC5989876
- DOI: 10.1002/jcsm.12288
The double-edged sword of endoplasmic reticulum stress in uremic sarcopenia through myogenesis perturbation
Abstract
Background: Sarcopenia is the age-related degeneration characterized with the decline of skeletal muscle mass, strength, and function. The imbalance of protein synthesis and degradation which jeopardizes immune, hormone regulation, and muscle-motor neuron connection is the main cause of sarcopenia. There is limited knowledge regarding molecular mechanism of sarcopenia. As the endoplasmic reticulum is the control centre of the protein syntheses and degradation, we hypothesized that endoplasmic reticulum stress and unfolded protein response (UPR) play an important in the development of sarcopenia. Understanding the sarcopenia molecular mechanisms may benefit the therapeutic diagnosis and treatment in the future.
Methods: Mouse myoblast C2C12 cells are exposed to designated time and concentration of indoxyl sulfate (IS), a uremic toxin of chronic kidney disease. The proliferation, differentiation, and the expression of atrogin 1 are examined. The protein and mRNA expression of IS treated-C2C12 cells are inspected to distinguish the role of ER stress and oxidative stress underlying the sarcopenia.
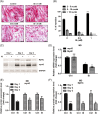
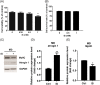
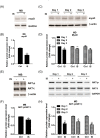
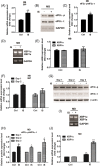
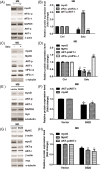
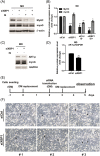
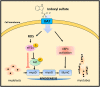
Results: Indoxyl sulfate inhibits myoblast differentiation. We demonstrate that as the number of multi-nuclei myotube decreased, the differentiation markers including myoD, myoG, and myosin heavy chain are also suppressed. Indoxyl sulfate inhibits myoblast proliferation and induces the myotubular atrophy marker atrogin-1 protein expression. Indoxyl sulfate stimulates eIF2α phosphorylation and XBP1 mRNA splicing in UPR. Interestingly, the oxidative stress is related to eIF2α phosphorylation but not XBP1 mRNA splicing. The eIF2α phosphorylation triggered by IS reduces myoD, myoG, and myosin heavy chain protein expression, which represents the anti-myogenic modulation on the early differentiation event. The XBP1 mRNA splicing induced by IS, however, is considered the adaptive response to restore the myogenic differentiation.
Conclusions: Our studies indicated that the ER stress and UPR modulation are critical in the chronic kidney disease uremic toxin-accumulated sarcopenia model. We believe that UPR-related signals showed great potential in clinical application.
Keywords: Chronic kidney disease; ER stress; Indoxyl sulfate; Myogenesis; Unfolded protein response.
© 2018 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of the Society on Sarcopenia, Cachexia and Wasting Disorders.
Figures

References
-
- Metter EJ, Conwit R, Tobin J, Fozard JL. Age‐associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci 1997;52:B267–B276. - PubMed
-
- Maggio M, Lauretani F, Ceda GP. Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr Metab Care 2013;16:3–13. - PubMed
-
- Messier V, Rabasa‐Lhoret R, Barbat‐Artigas S, Elisha B, Karelis AD, Aubertin‐Leheudre M. Menopause and sarcopenia: a potential role for sex hormones. Maturitas 2011;68:331–336. - PubMed
-
- Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol 2007;103:378–387. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

