Impact of the Oral Adsorbent AST-120 on Organ-Specific Accumulation of Uremic Toxins: LC-MS/MS and MS Imaging Techniques
- PMID: 29283413
- PMCID: PMC5793106
- DOI: 10.3390/toxins10010019
Impact of the Oral Adsorbent AST-120 on Organ-Specific Accumulation of Uremic Toxins: LC-MS/MS and MS Imaging Techniques
Abstract
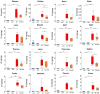
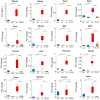
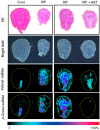
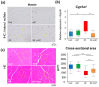
Elevated circulating uremic toxins are associated with a variety of symptoms and organ dysfunction observed in patients with chronic kidney disease (CKD). Indoxyl sulfate (IS) and p-cresyl sulfate (PCS) are representative uremic toxins that exert various harmful effects. We recently showed that IS induces metabolic alteration in skeletal muscle and causes sarcopenia in mice. However, whether organ-specific accumulation of IS and PCS is associated with tissue dysfunction is still unclear. We investigated the accumulation of IS and PCS using liquid chromatography/tandem mass spectrometry in various tissues from mice with adenine-induced CKD. IS and PCS accumulated in all 15 organs analyzed, including kidney, skeletal muscle, and brain. We also visualized the tissue accumulation of IS and PCS with immunohistochemistry and mass spectrometry imaging techniques. The oral adsorbent AST-120 prevented some tissue accumulation of IS and PCS. In skeletal muscle, reduced accumulation following AST-120 treatment resulted in the amelioration of renal failure-associated muscle atrophy. We conclude that uremic toxins can accumulate in various organs and that AST-120 may be useful in treating or preventing organ dysfunction in CKD, possibly by reducing tissue accumulation of uremic toxins.
Keywords: chronic kidney disease; indoxyl sulfate; mass spectrometry; p-cresyl sulfate; uremic toxin.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Saito S., Yisireyili M., Shimizu H., Ng H.Y., Niwa T. Indoxyl sulfate upregulates prorenin expression via nuclear factor-kappaB p65, signal transducer and activator of transcription 3, and reactive oxygen species in proximal tubular cells. J. Ren. Nutr. 2015;25:145–148. doi: 10.1053/j.jrn.2014.10.008. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

