Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder
- PMID: 27594980
- PMCID: PMC5009541
- DOI: 10.1186/s13229-016-0099-3
Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder
Abstract
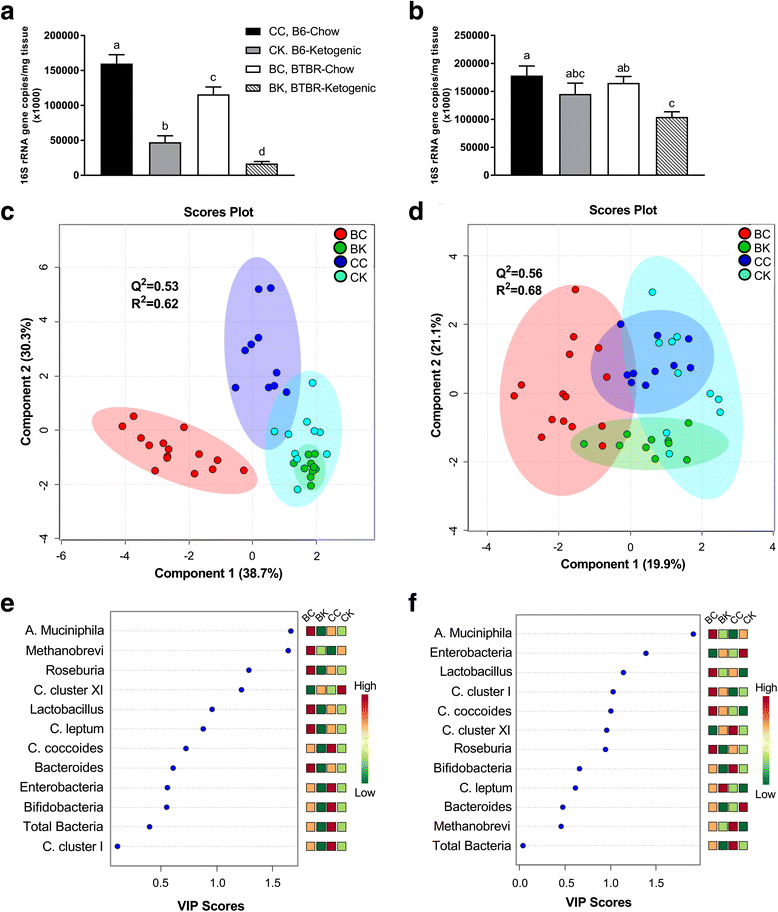
Background: Gastrointestinal dysfunction and gut microbial composition disturbances have been widely reported in autism spectrum disorder (ASD). This study examines whether gut microbiome disturbances are present in the BTBR(T + tf/j) (BTBR) mouse model of ASD and if the ketogenic diet, a diet previously shown to elicit therapeutic benefit in this mouse model, is capable of altering the profile.
Findings: Juvenile male C57BL/6 (B6) and BTBR mice were fed a standard chow (CH, 13 % kcal fat) or ketogenic diet (KD, 75 % kcal fat) for 10-14 days. Following diets, fecal and cecal samples were collected for analysis. Main findings are as follows: (1) gut microbiota compositions of cecal and fecal samples were altered in BTBR compared to control mice, indicating that this model may be of utility in understanding gut-brain interactions in ASD; (2) KD consumption caused an anti-microbial-like effect by significantly decreasing total host bacterial abundance in cecal and fecal matter; (3) specific to BTBR animals, the KD counteracted the common ASD phenotype of a low Firmicutes to Bacteroidetes ratio in both sample types; and (4) the KD reversed elevated Akkermansia muciniphila content in the cecal and fecal matter of BTBR animals.
Conclusions: Results indicate that consumption of a KD likely triggers reductions in total gut microbial counts and compositional remodeling in the BTBR mouse. These findings may explain, in part, the ability of a KD to mitigate some of the neurological symptoms associated with ASD in an animal model.
Keywords: Autism spectrum disorder; BTBR mouse; Gut microbiome; Ketogenic diet.
Figures
References
-
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One. 2013;8:e76993. doi: 10.1371/journal.pone.0076993. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

