Snus undermines quit attempts but not abstinence: a randomised clinical trial among US smokers
- PMID: 27071730
- PMCID: PMC5061602
- DOI: 10.1136/tobaccocontrol-2015-052783
Snus undermines quit attempts but not abstinence: a randomised clinical trial among US smokers
Abstract
Background: Observational studies and a few clinical trials suggest that use of low nitrosamine smokeless tobacco (snus) can facilitate smoking cessation. To better understand the real-world impact of snus on smoking behaviour, a large-scale, long-term clinical trial of naturalistic snus use among smokers is needed.
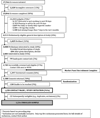
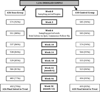
Study design: A nationwide clinical trial compared abstinence outcomes among smokers who were randomised to receive free samples of snus versus not. Participants (N=1236) were recruited throughout the US and assessed for 1 year following a 6-week naturalistic sampling period, with high retention throughout. Primary outcomes included self-reported quit attempts, floating abstinence (any 7-day period of non-smoking) and 7-day point-prevalence abstinence at 6 months and 12 months. Secondary outcomes were changes in smoking, motivation and confidence to quit and adverse events. No tobacco industry support was provided.
Results: Within snus group, 82% used at least once, and 16% were using regularly at end of sampling period. Compared to control participants, smokers in the snus group were less likely to make any quit attempt (RR=0.83; 95% CI 0.70 to 1.00), and any 24 h quit attempt (RR=0.77; 95% CI 0.63 to 0.95). There were no group differences on any measure of abstinence.
Conclusions: Provision of snus in a naturalistic context resulted in minimal uptake, and as a whole, undermined quit attempts and did not increase smoking abstinence. Results do not support the unguided, free provision of snus among smokers not motivated to quit as a means to facilitate quit attempts.
Trial registration number: NCT01509586, Results.
Keywords: Cessation; Harm Reduction; Non-cigarette tobacco products.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Drs. Alberg, Burris, Carpenter, Garrett-Mayer, Wahlquist report no conflicts of interests. Dr. Gray has received support from Merck Inc. and Supernus Pharmaceuticals for unrelated research. Dr. Cummings has received support from Pfizer for the evaluation of a hospital based cessation program, and has received payment as an expert witness in litigation against cigarette companies.
Figures

References
-
- Krautter GR, Chen PX, Borgerding MF. Consumption patterns and biomarkers of exposure in cigarette smokers switched to Snus, various dissovable tobacco products, Dual use, or tobacco abstinence. Regulatory Toxicology and Pharmacology. 2015;71:186–197. - PubMed
-
- Levy DT, Mumford EA, Cummings KM, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiology, Biomarkers & Prevention. 2004;13:2035–2042. - PubMed
-
- Levy DT, Mumford EA, Cummings KM, et al. The potential impact of a low-nitrosamine smokeless tobacco product on cigarette smoking in the United States: Estimates of a panel of experts. Addictive Behaviors. 2006;31:1190–1200. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
