Parabrachial Nucleus Contributions to Glucagon-Like Peptide-1 Receptor Agonist-Induced Hypophagia
- PMID: 25703200
- PMCID: PMC4839524
- DOI: 10.1038/npp.2015.50
Parabrachial Nucleus Contributions to Glucagon-Like Peptide-1 Receptor Agonist-Induced Hypophagia
Abstract
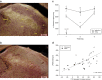
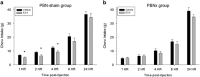
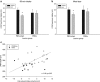
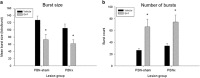
Exendin-4 (Ex4), a glucagon-like peptide-1 receptor (GLP-1R) agonist approved to treat type 2 diabetes mellitus, is well known to induce hypophagia in human and animal models. We evaluated the contributions of the hindbrain parabrachial nucleus (PBN) to systemic Ex4-induced hypophagia, as the PBN receives gustatory and visceral afferent relays and descending input from several brain nuclei associated with feeding. Rats with ibotenic-acid lesions targeted to the lateral PBN (PBNx) and sham controls received Ex4 (1 μg/kg) before 24 h home cage chow or 90 min 0.3 M sucrose access tests, and licking microstructure was analyzed to identify components of feeding behavior affected by Ex4. PBN lesion efficacy was confirmed using conditioned taste aversion (CTA) tests. As expected, sham control but not PBNx rats developed a CTA. In sham-lesioned rats, Ex4 reduced chow intake within 4 h of injection and sucrose intake within 90 min. PBNx rats did not show reduced chow or sucrose intake after Ex4 treatment, indicating that the PBN is necessary for Ex4 effects under the conditions tested. In sham-treated rats, Ex4 affected licking microstructure measures associated with hedonic taste evaluation, appetitive behavior, oromotor coordination, and inhibitory postingestive feedback. Licking microstructure responses in PBNx rats after Ex4 treatment were similar to sham-treated rats with the exception of inhibitory postingestive feedback measures. Together, the results suggest that the PBN critically contributes to the hypophagic effects of systemically delivered GLP-1R agonists by enhancing visceral feedback.
Figures



References
-
- Antin J, Gibbs J, Holt J, Young RC, Smith GP (1975). Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol 89: 784–790. - PubMed
-
- Asarian L, Corp ES, Hrupka B, Geary N (1998). Intracerebroventricular glucagon-like peptide-1 (7-36) amide inhibits sham feeding in rats without eliciting satiety. Physiol Behav 64: 367–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

