Effective Neutrophil Phagocytosis of Aspergillus fumigatus Is Mediated by Classical Pathway Complement Activation
- PMID: 25676601
- PMCID: PMC6738756
- DOI: 10.1159/000369493
Effective Neutrophil Phagocytosis of Aspergillus fumigatus Is Mediated by Classical Pathway Complement Activation
Abstract
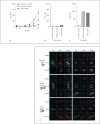
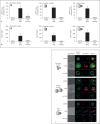
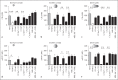
Aspergillus fumigatus is an important airborne fungal pathogen and a major cause of invasive fungal infections. Susceptible individuals become infected via the inhalation of dormant conidia. If the immune system fails to clear these conidia, they will swell, germinate and grow into large hyphal structures. Neutrophils are essential effector cells for controlling A. fumigatus infection. In general, opsonization of microbial particles is crucial for efficient phagocytosis and killing by neutrophils. Although the antibodies present in human serum do bind to all fungal morphotypes, we observed no direct antibody-mediated phagocytosis of A. fumigatus. We show that opsonization, phagocytosis and killing by neutrophils of A. fumigatus is complement-dependent. Using human sera depleted of key complement components, we investigated the contribution of the different complement initiation pathways in complement activation on the fungal surface. We describe the classical complement pathway as the main initiator of complement activation on A. fumigatus swollen conidia and germ tubes. Antibodies play an important role in complement activation and efficient innate recognition, phagocytosis and killing of A. fumigatus by neutrophils.
Figures


References
-
- Stevens DA, Melikian GL. Aspergillosis in the ‘nonimmunocompromised’ host. Immunol Invest. 2011;40:751–766. - PubMed
-
- O'Gorman CM, Fuller HT. Prevalence of culturable airborne spores of selected allergenic and pathogenic fungi in outdoor air. Atmospher Environ. 2008;42:4355–4368.
-
- Winkelstein JA, Marino MC, Johnston RB, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. - PubMed
-
- Young RC, Bennett JE, Vogel CL, Carbone PP, DeVita VT. Aspergillosis. The spectrum of the disease in 98 patients. Medicine (Baltimore) 1970;49:147–173. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

