Gut microbiota promote hematopoiesis to control bacterial infection
- PMID: 24629343
- PMCID: PMC4144825
- DOI: 10.1016/j.chom.2014.02.006
Gut microbiota promote hematopoiesis to control bacterial infection
Abstract
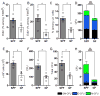
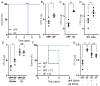
The commensal microbiota impacts specific immune cell populations and their functions at peripheral sites, such as gut mucosal tissues. However, it remains unknown whether gut microbiota control immunity through regulation of hematopoiesis at primary immune sites. We reveal that germ-free mice display reduced proportions and differentiation potential of specific myeloid cell progenitors of both yolk sac and bone marrow origin. Homeostatic innate immune defects may lead to impaired early responses to pathogens. Indeed, following systemic infection with Listeria monocytogenes, germ-free and oral-antibiotic-treated mice display increased pathogen burden and acute death. Recolonization of germ-free mice with a complex microbiota restores defects in myelopoiesis and resistance to Listeria. These findings reveal that gut bacteria direct innate immune cell development via promoting hematopoiesis, contributing to our appreciation of the deep evolutionary connection between mammals and their microbiota.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest related to this work.
Figures

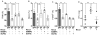
References
-
- Aichele P, Zinke J, Grode L, Schwendener RA, Kaufmann SH, Seiler P. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J Immunol. 2003;171:1148–1155. - PubMed
-
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. - PubMed
-
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. - PubMed
-
- Bugl S, Wirths S, Radsak MP, Schild H, Stein P, André MC, Müller MR, Malenke E, Wiesner T, Märklin M, et al. Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood. 2013;121:723–733. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

