SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress
- PMID: 24252090
- PMCID: PMC4085980
- DOI: 10.1089/ars.2013.5420
SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress
Abstract
Aims: Adenosine triphosphate (ATP) synthase uses chemiosmotic energy across the inner mitochondrial membrane to convert adenosine diphosphate and orthophosphate into ATP, whereas genetic deletion of Sirt3 decreases mitochondrial ATP levels. Here, we investigate the mechanistic connection between SIRT3 and energy homeostasis.
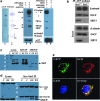
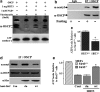
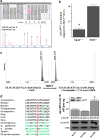
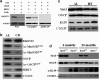
Results: By using both in vitro and in vivo experiments, we demonstrate that ATP synthase F1 proteins alpha, beta, gamma, and Oligomycin sensitivity-conferring protein (OSCP) contain SIRT3-specific reversible acetyl-lysines that are evolutionarily conserved and bind to SIRT3. OSCP was further investigated and lysine 139 is a nutrient-sensitive SIRT3-dependent deacetylation target. Site directed mutants demonstrate that OSCP(K139) directs, at least in part, mitochondrial ATP production and mice lacking Sirt3 exhibit decreased ATP muscle levels, increased ATP synthase protein acetylation, and an exercise-induced stress-deficient phenotype.
Innovation: This work connects the aging and nutrient response, via SIRT3 direction of the mitochondrial acetylome, to the regulation of mitochondrial energy homeostasis under nutrient-stress conditions by deacetylating ATP synthase proteins.
Conclusion: Our data suggest that acetylome signaling contributes to mitochondrial energy homeostasis by SIRT3-mediated deacetylation of ATP synthase proteins.
Figures
References
-
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, and Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325: 834–840, 2009 - PubMed
-
- Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P, Moullan N, Pirinen E, Yamamoto H, Houten SM, Schoonjans K, and Auwerx J. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep 2: 425, 2012 - PMC - PubMed
-
- Gius D, Botero A, Shah S, and Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol Lett 106: 93–106, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

