The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors
- PMID: 24105414
- PMCID: PMC3882373
- DOI: 10.1152/ajpendo.00413.2013
The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors
Abstract
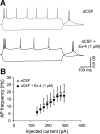
Glucagon-like peptide-1 receptor (GLP-1R) activation in the ventral tegmental area (VTA) is physiologically relevant for the control of palatable food intake. Here, we tested whether the food intake-suppressive effects of VTA GLP-1R activation are mediated by glutamatergic signaling within the VTA. Intra-VTA injections of the GLP-1R agonist exendin-4 (Ex-4) reduced palatable high-fat food intake in rats primarily by reducing meal size; these effects were mediated in part via glutamatergic AMPA/kainate but not NMDA receptor signaling. Additional behavioral data indicated that GLP-1R expressed specifically within the VTA can partially mediate the intake- and body weight-suppressive effects of systemically administered Ex-4, offering the intriguing possibility that this receptor population may be clinically relevant for food intake control. Intra-VTA Ex-4 rapidly increased tyrosine hydroxylase levels within the VTA, suggesting that GLP-1R activation modulates VTA dopaminergic signaling. Further evidence for this hypothesis was provided by electrophysiological data showing that Ex-4 increased the frequency of AMPA-mediated currents and reduced the paired/pulse ratio in VTA dopamine neurons. Together, these data provide novel mechanisms by which GLP-1R agonists in the mesolimbic reward system control for palatable food intake.
Keywords: 2-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid; diabetes; dopamine; glucagon-like peptide-1; glutamate; obesity; reward.
Figures
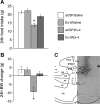
 main effect of CNQX (P < 0.05); #interaction between CNQX and Ex-4 (P < 0.05). Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.
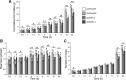
main effect of CNQX (P < 0.05); #interaction between CNQX and Ex-4 (P < 0.05). Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.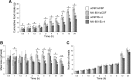
 main effect of MK-801 (P < 0.05); #statistically significant interaction between MK-801 and Ex-4. Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.
main effect of MK-801 (P < 0.05); #statistically significant interaction between MK-801 and Ex-4. Within time bin, bars with different letters are significantly different from each other (P < 0.05). All data are shown as means ± SE.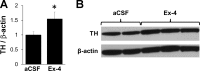

References
-
- Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev 28: 415–431, 2004 - PubMed
-
- Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374: 1606–1616, 2009 - PubMed
-
- Barbano MF, Le Saux M, Cador M. Involvement of dopamine and opioids in the motivation to eat: influence of palatability, homeostatic state, and behavioral paradigms. Psychopharmacology 203: 475–487, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

