Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults
- PMID: 23942319
- PMCID: PMC4010971
- DOI: 10.1038/ijo.2013.149
Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults
Abstract
Background: Liraglutide 3.0 mg, with diet and exercise, produced substantial weight loss over 1 year that was sustained over 2 years in obese non-diabetic adults. Nausea was the most frequent side effect.
Objective: To evaluate routinely collected data on nausea and vomiting among individuals on liraglutide and their influence on tolerability and body weight.
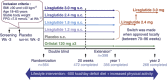
Design: A randomized, placebo-controlled, double-blind 20-week study with an 84-week extension (sponsor unblinded at 20 weeks, open-label after 1 year) in eight European countries (Clinicaltrials.gov: NCT00422058).
Subjects: After commencing a 500-kcal/day deficit diet plus exercise, 564 participants (18-65 years, body mass index (BMI) 30-40 kg m(-2)) were randomly assigned (after a 2-week run-in period) to once-daily subcutaneous liraglutide (1.2, 1.8, 2.4 or 3.0 mg), placebo or open-label orlistat (120 mg × 3 per day). After 1 year, participants on liraglutide/placebo switched to liraglutide 2.4 mg, and subsequently, to liraglutide 3.0 mg (based on 20-week and 1-year results, respectively).
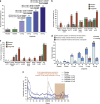
Results: The intention-to-treat population comprised 561 participants (n=90-98 per arm, age 45.9±10.3 years, BMI 34.8±2.7 kg m(-2) (mean±s.d.)). In year 1, more participants reported ⩾1 episode of nausea/vomiting on treatment with liraglutide 1.2-3.0 mg (17-38%) than with placebo or orlistat (both 4%, P⩽0.001). Most episodes occurred during dose escalation (weeks 1-6), with 'mild' or 'moderate' symptoms. Among participants on liraglutide 3.0 mg, 48% reported some nausea and 13% some vomiting, with considerable variation between countries, but only 4 out of 93 (4%) reported withdrawals. The mean 1-year weight loss on treatment with liraglutide 3.0 mg from randomization was 9.2 kg for participants reporting nausea/vomiting episodes, versus 6.3 kg for those with none (a treatment difference of 2.9 kg (95% confidence interval 0.5-5.3); P=0.02). Both weight losses were significantly greater than the respective weight losses for participants on placebo (P<0.001) or orlistat (P<0.05). Quality-of-life scores at 20 weeks improved similarly with or without nausea/vomiting on treatment with liraglutide 3.0 mg.
Conclusion: Transient nausea and vomiting on treatment with liraglutide 3.0 mg was associated with greater weight loss, although symptoms appeared tolerable and did not attenuate quality-of-life improvements. Improved data collection methods on nausea are warranted.
Figures


References
-
- Mayer MA, Hocht C, Puyo A, Taira CA. Recent advances in obesity pharmacotherapy. Curr Clin Pharmacol. 2009;4:53–61. - PubMed
-
- Murray CJL, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. - PubMed
-
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. J Am Med Assoc. 2007;298:2028–2037. - PubMed
-
- Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification: a 2-y follow-up study. Int J Obes Relat Metab Disord. 2003;27:1072–1080. - PubMed
-
- Hassan MK, Joshi AV, Madhavan SS, Amonkar MM. Obesity and health-related quality of life: a cross-sectional analysis of the US population. Int J Obes Relat Metab Disord. 2003;27:1227–1232. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

