NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer
- PMID: 23281400
- PMCID: PMC3561825
- DOI: 10.1172/JCI62236
NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer
Abstract
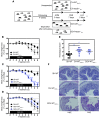
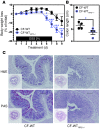
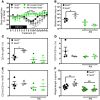
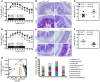
Instability in the composition of gut bacterial communities (dysbiosis) has been linked to common human intestinal disorders, such as Crohn's disease and colorectal cancer. Here, we show that dysbiosis caused by Nod2 deficiency gives rise to a reversible, communicable risk of colitis and colitis-associated carcinogenesis in mice. Loss of either Nod2 or RIP2 resulted in a proinflammatory microenvironment that enhanced epithelial dysplasia following chemically induced injury. The condition could be improved by treatment with antibiotics or an anti-interleukin-6 receptor-neutralizing antibody. Genotype-dependent disease risk was communicable via maternally transmitted microbiota in both Nod2-deficient and WT hosts. Furthermore, reciprocal microbiota transplantation reduced disease risk in Nod2-deficient mice and led to long-term changes in intestinal microbial communities. Conversely, disease risk was enhanced in WT hosts that were recolonized with dysbiotic fecal microbiota from Nod2-deficient mice. Thus, we demonstrated that licensing of dysbiotic microbiota is a critical component of disease risk. Our results demonstrate that NOD2 has an unexpected role in shaping a protective assembly of gut bacterial communities and suggest that manipulation of dysbiosis is a potential therapeutic approach in the treatment of human intestinal disorders.
Figures




Comment in
-
NOD2 protects against colorectal cancer via regulation of gut bacteria.Cancer Discov. 2013 Mar;3(3):OF17. doi: 10.1158/2159-8290.CD-RW2013-011. Epub 2013 Jan 17. Cancer Discov. 2013. PMID: 23475887
-
NOD2 prevents emergence of disease-predisposing microbiota.Gut Microbes. 2013 Jul-Aug;4(4):353-6. doi: 10.4161/gmic.25275. Epub 2013 Jun 6. Gut Microbes. 2013. PMID: 23778641 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

