A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease
- PMID: 23044924
- PMCID: PMC3535550
- DOI: 10.1002/art.37732
A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease
Abstract
Objective: Autoantigen presentation by HLA-DR molecules is thought to be a central component of many autoimmune diseases, but identifying disease-relevant autoantigens has been a difficult challenge. In this study we aimed to identify autoantigens in patients with antibiotic-refractory Lyme arthritis, in which infection-induced autoimmunity is thought to play an important role.
Methods: Using tandem mass spectrometry, naturally presented HLA-DR self peptides from a patient's synovium were identified, synthesized, and reacted with his peripheral blood mononuclear cells (PBMCs). Immunoreactive peptides and their source proteins were then tested for T and B cell responses using large numbers of patient cells or sera.
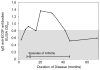
Results: Of 120 HLA-DR-presented self peptides identified from one patient, one peptide derived from endothelial cell growth factor (ECGF) caused his PBMCs to proliferate. T and B cell responses to ECGF occurred systemically in ∼10-30% of patients with early or late manifestations of Lyme disease, primarily in those with refractory arthritis-associated HLA-DR alleles, such as DRB1*0101 and 0401. Compared with patients with antibiotic-responsive arthritis, those with antibiotic-refractory arthritis had significantly higher concentrations of ECGF in synovial fluid (P<0.0001) and more often had ECGF antibody reactivity. Among non-antibiotic-treated historical patients who developed arthritis, 26% had ECGF reactivity, which often developed before the onset of arthritis and was associated with significantly longer courses of arthritis.
Conclusion: T and B cell responses to ECGF occur in a subset of patients with Lyme disease, particularly in those with antibiotic-refractory arthritis, providing the first direct evidence of autoimmune T and B cell responses in this illness.
Copyright © 2013 by the American College of Rheumatology.
Figures
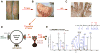




References
-
- Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med. 2011;365:1612–23. - PubMed
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. - PubMed
-
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. - PubMed
-
- Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 HV028178/HV/NHLBI NIH HHS/United States
- N01-HV-00239/HV/NHLBI NIH HHS/United States
- S10-RR-20946/RR/NCRR NIH HHS/United States
- HHSN268201000031C/HL/NHLBI NIH HHS/United States
- S10 RR020946/RR/NCRR NIH HHS/United States
- AR-20358/AR/NIAMS NIH HHS/United States
- N01 HV028178/HL/NHLBI NIH HHS/United States
- S10 RR015942/RR/NCRR NIH HHS/United States
- P41-GM-104603/RR-10888/GM/NIGMS NIH HHS/United States
- P41 GM104603/GM/NIGMS NIH HHS/United States
- S10-RR-15942/RR/NCRR NIH HHS/United States
- N01-HV-28178/HV/NHLBI NIH HHS/United States
- R01 AR020358/AR/NIAMS NIH HHS/United States
- P41 RR010888/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

