Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa
- PMID: 22605337
- PMCID: PMC3390633
- DOI: 10.1074/jbc.M112.375378
Structure of the cytoplasmic region of PelD, a degenerate diguanylate cyclase receptor that regulates exopolysaccharide production in Pseudomonas aeruginosa
Abstract
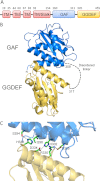
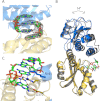
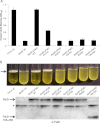
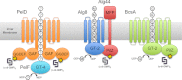
High cellular concentrations of bis-(3',5')-cyclic dimeric guanosine mono-phosphate (c-di-GMP) regulate a diverse range of phenotypes in bacteria including biofilm development. The opportunistic pathogen Pseudomonas aeruginosa produces the PEL polysaccharide to form a biofilm at the air-liquid interface of standing cultures. Among the proteins required for PEL polysaccharide production, PelD has been identified as a membrane-bound c-di-GMP-specific receptor. In this work, we present the x-ray crystal structure of a soluble cytoplasmic region of PelD in its apo and c-di-GMP complexed forms. The structure of PelD reveals an N-terminal GAF domain and a C-terminal degenerate GGDEF domain, the latter of which binds dimeric c-di-GMP at an RXXD motif that normally serves as an allosteric inhibition site for active diguanylate cyclases. Using isothermal titration calorimetry, we demonstrate that PelD binds c-di-GMP with low micromolar affinity and that mutation of residues involved in binding not only decreases the affinity of this interaction but also abrogates PEL-specific phenotypes in vivo. Bioinformatics analysis of the juxtamembrane region of PelD suggests that it contains an α-helical stalk region that connects the soluble region to the transmembrane domains and that similarly to other GAF domain containing proteins, this region likely forms a coiled-coil motif that mediates dimerization. PelD with Alg44 and BcsA of the alginate and cellulose secretion systems, respectively, collectively constitute a group of c-di-GMP receptors that appear to regulate exopolysaccharide assembly at the protein level through activation of their associated glycosyl transferases.
Figures
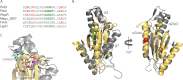

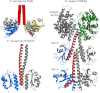
References
-
- Merighi M., Lee V. T., Hyodo M., Hayakawa Y., Lory S. (2007) The second messenger bis-(3′-5′)-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol. Microbiol. 65, 876–895 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

