Modulating intestinal immune responses by lipoteichoic acid-deficient Lactobacillus acidophilus
- PMID: 22339459
- PMCID: PMC3286340
- DOI: 10.2217/imt.11.163
Modulating intestinal immune responses by lipoteichoic acid-deficient Lactobacillus acidophilus
Abstract
Aim: To investigate the mechanism(s) by which the intestinal commensal microbe Lactobacillus acidophilus can affect host immunity, we studied the role of a component of the cell wall, lipoteichoic acid, in colitis.
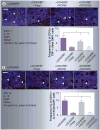
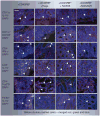
Materials & methods: Colitis was induced by the intraperitoneal injection of pathogenic CD4(+)CD25(-)CD45RB(hi) T cells into Rag1(-/-) mice. The parental strain, NCK56, or the lipoteichoic acid-deficient strain, NCK2025, was then administered orally. Fluorescent microscopy was employed to examine resulting cell populations and their cytokine production in the colon.
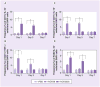
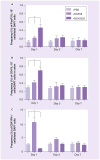
Results: NCK2025 enhanced IL-10 production by dendritic cells and macrophages. Increased numbers of regulatory dendritic cells coincided with the induction of activated FoxP3(+) Tregs.
Conclusion: These results suggest that the oral administration of the genetically modified strain NCK2025 may be an effective immunotherapeutic approach that reprograms the immune response in colonic inflammatory conditions.
Figures

References
-
- Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3(4):331–341. - PubMed
-
- MacDonald TT, Gordon JN. Bacterial regulation of intestinal immune responses. Gastroenterol Clin North Am. 2005;34(3):401–412. VII–VIII. - PubMed
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. - PubMed
-
- Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369(9573):1627–1640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
