Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity
- PMID: 22040113
- PMCID: PMC3237733
- DOI: 10.1111/j.1365-2958.2011.07905.x
Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity
Abstract
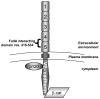
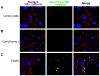
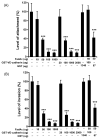
Fusobacterium nucleatum is a Gram-negative oral anaerobe, capable of systemic dissemination causing infections and abscesses, often in mixed-species, at different body sites. We have shown previously that F. nucleatum adheres to and invades host epithelial and endothelial cells via a novel FadA adhesin. In this study, vascular endothelial (VE)-cadherin, a member of the cadherin family and a cell-cell junction molecule, was identified as the endothelial receptor for FadA, required for F. nucleatum binding to the cells. FadA colocalized with VE-cadherin on endothelial cells, causing relocation of VE-cadherin away from the cell-cell junctions. As a result, the endothelial permeability was increased, allowing the bacteria to cross the endothelium through loosened junctions. This crossing mechanism may explain why the organism is able to disseminate systemically to colonize in different body sites and even overcome the placental and blood-brain barriers. Co-incubation of F. nucleatum and Escherichia coli enhanced penetration of the endothelial cells by the latter in the transwell assays, suggesting F. nucleatum may serve as an 'enabler' for other microorganisms to spread systemically. This may explain why F. nucleatum is often found in mixed infections. This study reveals a possible novel dissemination mechanism utilized by pathogens.
© 2011 Blackwell Publishing Ltd.
Figures

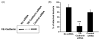

References
-
- Ahrens T, Lambert M, Pertz O, Sasaki T, Schulthess T, Mege RM, Timpl R, Engel J. Homoassociation of VE-cadherin follows a mechanism common to “classical” cadherins. J Mol Biol. 2003;325:733–742. - PubMed
-
- Bertrand B, Wakabayashi S, Ikeda T, Pouyssegur J, Shigekawa M. The Na+/H+ exchanger isoform 1 (NHE1) is a novel member of the calmodulin-binding proteins. Identification and characterization of calmodulin-binding sites. J Biol Chem. 1994;269:13703–13709. - PubMed
-
- Bonazzi M, Veiga E, Pizarro-Cerda J, Cossart P. Successive post-translational modifications of E-cadherin are required for InlA-mediated internalization of Listeria monocytogenes. Cell Microbiol. 2008;10:2208–2222. - PubMed
-
- Bulla R, Villa A, Bossi F, Cassetti A, Radillo O, Spessotto P, De Seta F, Guaschino S, Tedesco F. VE-cadherin is a critical molecule for trophoblast-endothelial cell interaction in decidual spiral arteries. Exp Cell Res. 2005;303:101–113. - PubMed
-
- Cheung WY, Bellas J. Case report: Lemierre syndrome presenting with fever and pharyngitis. Am Fam Physician. 2007a;75:979–980. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

