High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production
- PMID: 21386058
- PMCID: PMC3118596
- DOI: 10.1152/ajpendo.00666.2010
High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production
Abstract
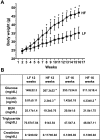
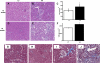
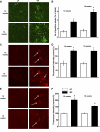
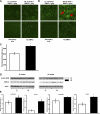
Obesity and metabolic syndrome are associated with an increased risk for several diabetic complications, including diabetic nephropathy and chronic kidney diseases. Oxidative stress and mitochondrial dysfunction are often proposed mechanisms in various organs in obesity models, but limited data are available on the kidney. Here, we fed a lard-based high-fat diet to mice to investigate structural changes, cellular and subcellular oxidative stress and redox status, and mitochondrial biogenesis and function in the kidney. The diet induced characteristic changes, including glomerular hypertrophy, fibrosis, and interstitial scarring, which were accompanied by a proinflammatory transition. We demonstrate evidence for oxidative stress in the kidney through 3-nitrotyrosine and protein radical formation on high-fat diet with a contribution from iNOS and NOX-4 as well as increased generation of mitochondrial oxidants on carbohydrate- and lipid-based substrates. The increased H(2)O(2) emission in the mitochondria suggests altered redox balance and mitochondrial ROS generation, contributing to the overall oxidative stress. No major derailments were observed in respiratory function or biogenesis, indicating preserved and initially improved bioenergetic parameters and energy production. We suggest that, regardless of the oxidative stress events, the kidney developed an adaptation to maintain normal respiratory function as a possible response to an increased lipid overload. These findings provide new insights into the complex role of oxidative stress and mitochondrial redox status in the pathogenesis of the kidney in obesity and indicate that early oxidative stress-related changes, but not mitochondrial bioenergetic dysfunction, may contribute to the pathogenesis and development of obesity-linked chronic kidney diseases.
Figures


References
-
- Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol 24: 46–53, 2004 - PubMed
-
- Abrass CK. Overview: obesity: what does it have to do with kidney disease? J Am Soc Nephrol 15: 2768–2772, 2004 - PubMed
-
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009 - PMC - PubMed
-
- Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257–31266, 2007 - PubMed
-
- Bagby SP. Obesity-initiated metabolic syndrome and the kidney: a recipe for chronic kidney disease? J Am Soc Nephrol 15: 2775–2791, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

