Aureusimines in Staphylococcus aureus are not involved in virulence
- PMID: 21209955
- PMCID: PMC3012096
- DOI: 10.1371/journal.pone.0015703
Aureusimines in Staphylococcus aureus are not involved in virulence
Abstract
Background: Recently, dipeptide aureusimines were reported to activate expression of staphylococcal virulence genes, such as alpha-hemolysin, and increase S. aureus virulence. Surprisingly, most of the virulence genes affected by aureusimines form part of the regulon of the SaeRS two component system (TCS), raising the possibility that SaeRS might be directly or indirectly involved in the aureusimine-dependent signaling process.
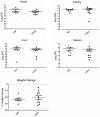
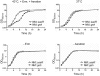
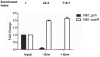
Methodology/principal findings: Using HPLC analyses, we confirmed that a transposon mutant of ausA, the gene encoding the aureusimine dipeptide synthesis enzyme, does not produce dipeptides. However, the transposon mutant showed normal hemolysis activity and alpha-hemolysin/SaeP production. Furthermore, the P1 promoter of the sae operon, one of the targets of the SaeRS TCS, showed normal transcription activity. Moreover, in contrast to the original report, the ausA transposon mutant did not exhibit attenuated virulence in an animal infection model. DNA sequencing revealed that the ausA deletion mutant used in the original study has an 83 nt-duplication in saeS. Hemolysis activity of the original mutant was restored by a plasmid carrying the sae operon. A mutant of the sae operon showed elevated resistance to chloramphenicol and erythromycin, two antibiotics widely used during staphylococcal mutagenesis. At 43°C in the presence of erythromycin and aeration, the conditions typically employed for staphylococcal mutagenesis, an saeR transposon mutant grew much faster than a control mutant and the saeR mutant was highly enriched in a mixed culture experiment.
Conclusions/significance: Our results show that the previously reported roles of aureusimines in staphylococcal gene regulation and virulence were due to an unintended mutation in saeS, which was likely selected due to elevated resistance of the mutant to environmental stresses. Thus, there is no evidence indicating that the dipeptide aureusimines play a role in sae-mediated virulence factor production or contribute to staphylococcal virulence.
Conflict of interest statement
Figures




References
-
- Archer GL. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis. 1998;26:1179–1181. - PubMed
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. - PubMed
-
- Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. - PubMed
-
- Giraudo AT, Calzolari A, Cataldi AA, Bogni C, Nagel R. The sae locus of Staphylococcus aureus encodes a two-component regulatory system. FEMS Microbiol Lett. 1999;177:15–22. - PubMed
-
- Giraudo AT, Cheung AL, Nagel R. The sae locus of Staphylococcus aureus controls exoprotein synthesis at the transcriptional level. Arch Microbiol. 1997;168:53–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

