Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD)
- PMID: 19289857
- PMCID: PMC2699702
- DOI: 10.2337/dc08-2124
Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD)
Erratum in
- Diabetes Care. 2010 Mar;33(3):692
Abstract
Objective: To determine the efficacy and safety of liraglutide (a glucagon-like peptide-1 receptor agonist) when added to metformin and rosiglitazone in type 2 diabetes.
Research design and methods: This 26-week, double-blind, placebo-controlled, parallel-group trial randomized 533 subjects (1:1:1) to once-daily liraglutide (1.2 or 1.8 mg) or liraglutide placebo in combination with metformin (1 g twice daily) and rosiglitazone (4 mg twice daily). Subjects had type 2 diabetes, A1C 7-11% (previous oral antidiabetes drug [OAD] monotherapy >or=3 months) or 7-10% (previous OAD combination therapy >or=3 months), and BMI <or=45 kg/m(2).
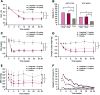
Results: Mean A1C values decreased significantly more in the liraglutide groups versus placebo (mean +/- SE -1.5 +/- 0.1% for both 1.2 and 1.8 mg liraglutide and -0.5 +/- 0.1% for placebo). Fasting plasma glucose decreased by 40, 44, and 8 mg/dl for 1.2 and 1.8 mg and placebo, respectively, and 90-min postprandial glucose decreased by 47, 49, and 14 mg/dl, respectively (P < 0.001 for all liraglutide groups vs. placebo). Dose-dependent weight loss occurred with 1.2 and 1.8 mg liraglutide (1.0 +/- 0.3 and 2.0 +/- 0.3 kg, respectively) (P < 0.0001) compared with weight gain with placebo (0.6 +/- 0.3 kg). Systolic blood pressure decreased by 6.7, 5.6, and 1.1 mmHg with 1.2 and 1.8 mg liraglutide and placebo, respectively. Significant increases in C-peptide and homeostasis model assessment of beta-cell function and significant decreases in the proinsulin-to-insulin ratio occurred with liraglutide versus placebo. Minor hypoglycemia occurred more frequently with liraglutide, but there was no major hypoglycemia. Gastrointestinal adverse events were more common with liraglutide, but most occurred early and were transient.
Conclusions: Liraglutide combined with metformin and a thiazolidinedione is a well-tolerated combination therapy for type 2 diabetes, providing significant improvements in glycemic control.
Trial registration: ClinicalTrials.gov NCT00333151.
Figures
References
-
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: the ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–43 - PubMed
-
- UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53 - PubMed
-
- Green J, Feinglos M: Update on type 2 diabetes mellitus: understanding changes in the diabetes treatment paradigm. Int J Clin Pract 2007;61(Suppl. 154):3–11 - PubMed
-
- Holst JJ: The physiology of glucagon-like peptide 1. Physiol Rev 2007;87:1409–1439 - PubMed
-
- Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H: Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 2000;43:1664–1669 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

