Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus
- PMID: 18987209
- PMCID: PMC2585803
- DOI: 10.1523/JNEUROSCI.3419-08.2008
Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus
Abstract
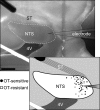
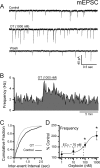
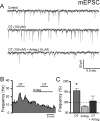
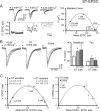
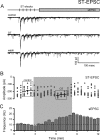
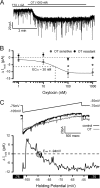
Cranial visceral afferents travel via the solitary tract (ST) to contact neurons within the ST nucleus (NTS) and activate homeostatic reflexes. Hypothalamic projections from the paraventricular nucleus (PVN) release oxytocin (OT) to modulate visceral afferent communication with NTS neurons. However, the cellular mechanisms through which OT acts are poorly understood. Here, we electrophysiologically identified second-order NTS neurons in horizontal brainstem slices by their low-jitter, ST-evoked glutamatergic EPSCs. OT increased the frequency of miniature EPSCs in half of the NTS second-order neurons (13/24) but did not alter event kinetics or amplitudes. These actions were blocked by a selective OT receptor antagonist. OT increased the amplitude of ST-evoked EPSCs with no effect on event kinetics. Variance-mean analysis of ST-evoked EPSCs indicated OT selectively increased the release probability of glutamate from the ST afferent terminals. In OT-sensitive neurons, OT evoked an inward holding current and increased input resistance. The OT-sensitive current reversed at the K(+) equilibrium potential. In in vivo studies, NTS neurons excited by vagal cardiopulmonary afferents were juxtacellularly labeled with Neurobiotin and sections were stained to show filled neurons and OT-immunoreactive axons. Half of these physiologically characterized neurons (5/10) showed close appositions by OT fibers consistent with synaptic contacts. Electron microscopy of medial NTS found immunoreactive OT within synaptic boutons. Together, these findings suggest that OT released from PVN axons acts on a subset of second-order neurons within medial NTS to enhance visceral afferent transmission via presynaptic and postsynaptic mechanisms.
Figures


References
-
- Andresen MC, Kunze DL. Nucleus tractus solitarius: gateway to neural circulatory control. Annu Rev Physiol. 1994;56:93–116. - PubMed
-
- Andresen MC, Yang MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol. 1990;259:H1307–H1311. - PubMed
-
- Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
