The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis
- PMID: 18162536
- PMCID: PMC2224176
- DOI: 10.1073/pnas.0710685105
The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis
Abstract
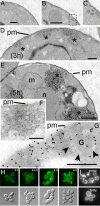
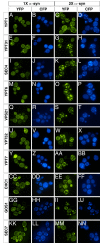
alpha-Synuclein (alpha-syn), a protein of unknown function, is the most abundant protein in Lewy bodies, the histological hallmark of Parkinson's disease (PD). In yeast alpha-syn inhibits endoplasmic reticulum (ER)-to-Golgi (ER-->Golgi) vesicle trafficking, which is rescued by overexpression of a Rab GTPase that regulates ER-->Golgi trafficking. The homologous Rab1 rescues alpha-syn toxicity in dopaminergic neuronal models of PD. Here we investigate this conserved feature of alpha-syn pathobiology. In a cell-free system with purified transport factors alpha-syn inhibited ER-->Golgi trafficking in an alpha-syn dose-dependent manner. Vesicles budded efficiently from the ER, but their docking or fusion to Golgi membranes was inhibited. Thus, the in vivo trafficking problem is due to a direct effect of alpha-syn on the transport machinery. By ultrastructural analysis the earliest in vivo defect was an accumulation of morphologically undocked vesicles, starting near the plasma membrane and growing into massive intracellular vesicular clusters in a dose-dependent manner. By immunofluorescence/immunoelectron microscopy, these clusters were associated both with alpha-syn and with diverse vesicle markers, suggesting that alpha-syn can impair multiple trafficking steps. Other Rabs did not ameliorate alpha-syn toxicity in yeast, but RAB3A, which is highly expressed in neurons and localized to presynaptic termini, and RAB8A, which is localized to post-Golgi vesicles, suppressed toxicity in neuronal models of PD. Thus, alpha-syn causes general defects in vesicle trafficking, to which dopaminergic neurons are especially sensitive.
Conflict of interest statement
Conflict of interest statement: S.L. is a cofounder of and owns stock in FoldRx Pharmaceuticals, a company developing therapies for diseases of protein misfolding, including Parkinson's disease. A.D.G. and S.L. are inventors on patents and patent applications that have been licensed to FoldRx Pharmaceuticals. J.-C.R. receives funds from FoldRx Pharmaceuticals as part of a compound-testing agreement between the company and his laboratory.
Figures
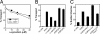
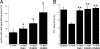
References
-
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha-synuclein: New targets for drug discovery. Neuron. 2006;52:33–38. - PubMed
-
- Spillantini MG, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum Mol Genet. 2006;15:3012–3023. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

