Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease
- PMID: 17251419
- PMCID: PMC6672917
- DOI: 10.1523/JNEUROSCI.3501-06.2007
Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease
Abstract
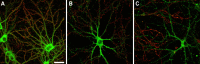
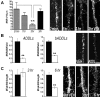
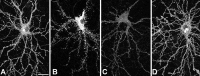
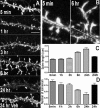
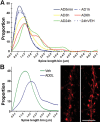
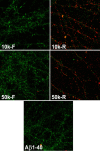
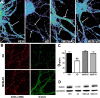
The basis for memory loss in early Alzheimer's disease (AD) seems likely to involve synaptic damage caused by soluble Abeta-derived oligomers (ADDLs). ADDLs have been shown to build up in the brain and CSF of AD patients and are known to interfere with mechanisms of synaptic plasticity, acting as gain-of-function ligands that attach to synapses. Because of the correlation between AD dementia and synaptic degeneration, we investigated here the ability of ADDLs to affect synapse composition, structure, and abundance. Using highly differentiated cultures of hippocampal neurons, a preferred model for studies of synapse cell biology, we found that ADDLs bound to neurons with specificity, attaching to presumed excitatory pyramidal neurons but not GABAergic neurons. Fractionation of ADDLs bound to forebrain synaptosomes showed association with postsynaptic density complexes containing NMDA receptors, consistent with observed attachment of ADDLs to dendritic spines. During binding to hippocampal neurons, ADDLs promoted a rapid decrease in membrane expression of memory-related receptors (NMDA and EphB2). Continued exposure resulted in abnormal spine morphology, with induction of long thin spines reminiscent of the morphology found in mental retardation, deafferentation, and prionoses. Ultimately, ADDLs caused a significant decrease in spine density. Synaptic deterioration, which was accompanied by decreased levels of the spine cytoskeletal protein drebrin, was blocked by the Alzheimer's therapeutic drug Namenda. The observed disruption of dendritic spines links ADDLs to a major facet of AD pathology, providing strong evidence that ADDLs in AD brain cause neuropil damage believed to underlie dementia.
Figures


References
-
- Aoki C, Sekino Y, Hanamura K, Fujisawa S, Mahadomrongkul V, Ren Y, Shirao T. Drebrin A is a postsynaptic protein that localizes in vivo to the submembranous surface of dendritic sites forming excitatory synapses. J Comp Neurol. 2005;483:383–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
