NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling
- PMID: 16581769
- PMCID: PMC1446931
- DOI: 10.1128/MCB.26.8.2936-2946.2006
NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling
Abstract
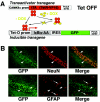
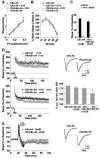
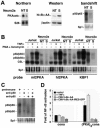
Synaptic activity-dependent de novo gene transcription is crucial for long-lasting neuronal plasticity and long-term memory. In a forebrain neuronal conditional NF-kappaB-deficient mouse model, we demonstrate here that the transcription factor NF-kappaB regulates spatial memory formation, synaptic transmission, and plasticity. Gene profiling experiments and analysis of regulatory regions identified the alpha catalytic subunit of protein kinase A (PKA), an essential memory regulator, as a new NF-kappaB target gene. Consequently, NF-kappaB inhibition led to a decrease in forskolin-induced CREB phosphorylation. Collectively, these results disclose a novel hierarchical transcriptional network involving NF-kappaB, PKA, and CREB that leads to concerted nuclear transduction of synaptic signals in neurons, accounting for the critical function of NF-kappaB in learning and memory.
Figures

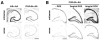

References
-
- Abel, T., P. V. Nguyen, M. Barad, T. A. Deuel, E. R. Kandel, and R. Bourtchouladze. 1997. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88:615-626. - PubMed
-
- Albensi, B. C., and M. P. Mattson. 2000. Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse 35:151-159. - PubMed
-
- Barco, A., J. M. Alarcon, and E. R. Kandel. 2002. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108:689-703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
