Rare genetic mutations shed light on the pathogenesis of Parkinson disease
- PMID: 12531866
- PMCID: PMC151882
- DOI: 10.1172/JCI17575
Rare genetic mutations shed light on the pathogenesis of Parkinson disease
Figures
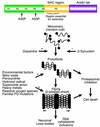
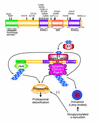
References
-
- Lang AE, Lozano AM. Parkinson’s disease. Second of two parts. N. Engl. J. Med. 1998;339:1130–1143. - PubMed
-
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N. Engl. J. Med. 1998;339:1044–1053. - PubMed
-
- Forno LS. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996;55:259–272. - PubMed
-
- Gasser T. Genetics of Parkinson’s disease. J. Neurol. 2001;248:833–840. - PubMed
-
- Roses AD. Apolipoprotein E and Alzheimer’s disease. The tip of the susceptibility iceberg. Ann. NY Acad. Sci. 1998;855:738–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

