alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins
- PMID: 11481478
- PMCID: PMC55381
- DOI: 10.1073/pnas.171300598
alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins
Abstract
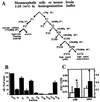
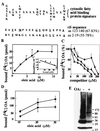
alpha-Synuclein (alphaS) is a 140-residue neuronal protein that forms insoluble cytoplasmic aggregates in Parkinson's disease (PD) and several other neurodegenerative disorders. Two missense mutations (A53T and A30P) are linked to rare forms of familial PD. The normal function of alphaS is unknown, and cultured cell systems that model its modification from soluble monomers to aggregated forms have not been reported. Through a systematic centrifugal fractionation of mesencephalic neuronal cell lines and transgenic mouse brains expressing wild-type or A53T human alphaS, we observed unusual, previously unrecognized species of alphaS that migrate well above the 17-kDa monomeric form in denaturing gels. Incubation at 65 degrees C of high-speed cytosols from cells or brains revealed a modified alphaS species migrating at approximately 36 kDa and an extensive higher molecular mass alphaS-reactive smear. Extraction of the cytosols with chloroform/methanol or with a resin (Lipidex 1000) that binds fatty acids resulted in a similar pattern of higher molecular mass alphaS forms. On the basis of this effect of delipidation, we reexamined the primary structure of alphaS and detected a motif at the N and C termini that is homologous to a fatty acid-binding protein signature. In accord, we found that purified human alphaS binds oleic acid, with an apparent K(d) of 12.5 microM. We also observed an enhanced association of A53T alphaS with microsomal membranes in both mesencephalic cells and transgenic mouse brains. We conclude that alphaS has biochemical properties and a structural motif that suggest it is a novel member of the fatty acid-binding protein family and may thus transport fatty acids between the aqueous and membrane phospholipid compartments of the neuronal cytoplasm.
Figures


References
-
- Lansbury P T. Neuron. 1997;19:1151–1154. - PubMed
-
- Spillantini M G, Schmidt M L, Lee V M-Y, Trojanowski J Q, Jakes R, Goedert M. Nature (London) 1997;388:389–390. - PubMed
-
- Polymeropoulos M H, Lavedan C, Leroy E, Ide S E, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, et al. Science. 1997;276:2045–2047. - PubMed
-
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen J T, Schols L, Riess O. Nat Genet. 1998;18:106–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

