An ongoing Candida auris outbreak in the New York metropolitan area is the largest recorded to date in North America. Laboratory surveillance revealed NY C. auris isolates are resistant to fluconazole, with variable resistance to other currently used broad-spectrum antifungal drugs, and that several isolates are panresistant. Thus, there is an urgent need for new drugs with a novel mechanism of action to combat the resistance challenge. Manogepix (MGX) is a first-in-class agent that targets the fungal Gwt1 enzyme.
KEYWORDS: APX001, fosmanogepix, APX001A, manogepix, Gwt1, antifungal, Candida auris, resistance
ABSTRACT
An ongoing Candida auris outbreak in the New York metropolitan area is the largest recorded to date in North America. Laboratory surveillance revealed NY C. auris isolates are resistant to fluconazole, with variable resistance to other currently used broad-spectrum antifungal drugs, and that several isolates are panresistant. Thus, there is an urgent need for new drugs with a novel mechanism of action to combat the resistance challenge. Manogepix (MGX) is a first-in-class agent that targets the fungal Gwt1 enzyme. The prodrug fosmanogepix is currently in phase 2 clinical development for the treatment of fungal infections. We evaluated the susceptibility of 200 New York C. auris isolates to MGX and 10 comparator drugs using CLSI methodology. MGX demonstrated lower MICs than comparators (MIC50 and MIC90, 0.03 mg/liter; range, 0.004 to 0.06 mg/liter). The local epidemiological cutoff value (ECV) for MGX indicated all C. auris isolates were within the population of wild-type (WT) strains; 0.06 mg/liter defines the upper limit of wild type (UL-WT). MGX was 8- to 32-fold more active than the echinocandins, 16- to 64-fold more active than the azoles, and 64-fold more active than amphotericin B. No differences were found in the MGX or comparators’ MIC50, MIC90, or geometric mean (GM) values when subsets of clinical, surveillance, and environmental isolates were evaluated. The range of MGX MIC values for six C. auris panresistant isolates was 0.008 to 0.015 mg/liter, and the median and mode MIC values were 0.015 mg/liter, demonstrating that MGX retains activity against these isolates. These data support further clinical evaluation of fosmanogepix for the treatment of C. auris infections, including highly resistant isolates.
INTRODUCTION
The yeast pathogen Candida auris has been shown to be responsible for severe illnesses among hospitalized patients in the New York metropolitan area (1). Of 1,092 U.S. C. auris clinical cases tracked by the CDC, 661 (60.53%) were reported from New York and New Jersey (https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html). C. auris cases in New York are concentrated among hospitalized patients and nursing home residents, with 504 clinical cases and 747 screening cases confirmed as of 15 May 2020 (https://www.health.ny.gov/diseases/communicable/c_auris/). An earlier analysis of New York C. auris cases indicated 23 (45%) of the 51 clinical case patients died within 90 days (1, 2). Initial reports suggested that C. auris emerged independently in four regions, namely, South Asia, East Asia, Africa, and South America, defined by clades I, II, II, and IV, respectively (3), with Iranian clade V arising more recently (4). An analysis of a diverse collection of C. auris isolates showed that clade I had the highest percentage of fluconazole (FLC)-resistant, amphotericin B (AMB)-resistant, multidrug-resistant (MDR), and extensively drug-resistant (XDR) isolates (5). C. auris isolates from New York are resistant to FLC, and many strains also demonstrated elevated MICs to voriconazole (VRC), AMB, flucytosine (5FC), and echinocandins, consistent with the finding that they were largely members of clade I (6). More recently, panresistant C. auris isolates (resistant to two or more azoles, AMB, and echinocandins) were recorded in New York (7). Clinicians and public health professionals face serious challenges in dealing with the large, sustained outbreak of C. auris in New York that is exacerbated by the limited availability of effective treatment options (1, 8). Thus, there is an urgent need to evaluate the efficacy of new or repurposed antifungal drugs against drug-resistant C. auris.
Manogepix (MGX; APX001A) is a first-in-class small-molecule antifungal agent that targets the fungal inositol acylase (Gwt1), which catalyzes an early step in the glycosylphosphatidylinositol (GPI)-anchor biosynthesis pathway (9–11). Inhibition of Gwt1 prevents the appropriate localization of cell wall mannoproteins, which compromises cell wall integrity, biofilm formation, and germ tube formation and results in severe fungal growth defects (12, 13). The phosphatidylinositol glycan anchor biosynthesis class W (PIGW) protein is the closest mammalian ortholog of the fungal Gwt1 protein and is not inhibited by MGX (12). Fosmanogepix (APX001), the N-phosphonooxymethyl prodrug of the active moiety MGX, is currently in clinical trials for the treatment of invasive fungal infections caused by Candida sp., including C. auris, Aspergillus sp., and rare molds (14, 15).
Several studies have previously evaluated the activity of MGX against worldwide or Indian collections of C. auris strains using both CLSI and EUCAST methodologies (16–19). MGX also demonstrates excellent activity against other Candida spp., with the exception of Candida krusei, as well as activity against Aspergillus, Scedosporium, Fusarium, and Lomentospora sp. and members of the Mucorales order (20–22). In addition, MGX retains activity against AMB- and azole-resistant strains of Aspergillus spp. and azole- and echinocandin-resistant Candida spp. (21–23). Efficacy studies in a wide range of yeast and mold species demonstrated significant improvements in both survival as well as CFU endpoints in lung, kidney, and brain, consistent with wide tissue distribution (17, 23–26).
The present study describes the in vitro activity of MGX and 10 comparators against a collection of 200 clinical, environmental, and surveillance isolates of C. auris collected in New York between 2017 and 2020, with the majority (94%) of isolates collected between 2018 and 2019 (6).
RESULTS
The MGX antifungal susceptibility profile of NY C. auris isolates compared with 10 other antifungals is summarized in Tables 1, 2, and 3. All 200 NY C. auris isolates were classified as resistant to FLC with MIC values of ≥32 mg/liter. A comparison of MIC90 values shows that MGX was 8- to 32-fold more active than the echinocandins anidulafungin (AFG), caspofungin (CAS), and micafungin (MFG); 16- to 64-fold more active than the azoles isavuconazole (ISA), itraconazole (ITC), posaconazole (POS), and VRC; 64-fold more active than AMB; and >1,000-fold more active than 5FC (Table 1).
TABLE 1.
Summary of the susceptibility of 200 NY C. auris isolatesa
| Antifungal | GM | Mode | MIC50 | MIC90 | Range |
|---|---|---|---|---|---|
| MGX | 0.02 | 0.03 | 0.03 | 0.03 | 0.004 to 0.06 |
| AFG | 0.33 | 0.50 | 0.25 | 1.0 | 0.03 to 8 |
| CAS | 0.14 | 0.25 | 0.12 | 0.25 | 0.016 to >16 |
| MFG | 0.16 | 0.12 | 0.12 | 0.25 | 0.06 to 4 |
| FLC | 246 | 256 | 256 | 256 | 32 to >256 |
| ISA | 0.71 | 1 | 1 | 1.0 | 0.03 to 2 |
| ITC | 0.58 | 1 | 0.5 | 1.0 | 0.125 to 1 |
| POS | 0.20 | 0.25 | 0.25 | 0.5 | 0.03 to 1 |
| VRC | 1.67 | 2 | 2 | 2 | 0.06 to 4 |
| AMB | 1.35 | 2 | 1 | 2 | 0.125 to 32 |
| 5FC | 0.14 | 0.06 | 0.064 | 32 | 0.023 to 32 |
All values are mg/liter.
TABLE 2.
Susceptibility of antifungal agents in clinical, surveillance, and environmental C. auris isolates
| Parameters by isolate type (n) | MIC (mg/liter) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FLC | VRC | ITC | ISA | POS | AFG | CAS | MFG | AMB | 5FC | MGX | |
| Clinical (85) | |||||||||||
| GM | 246 | 1.81 | 0.62 | 0.76 | 0.22 | 0.37 | 0.16 | 0.16 | 1.43 | 0.12 | 0.02 |
| Mode | 256 | 2 | 1 | 1 | 0.25 | 0.5 | 0.25 | 0.12 | 2 | 0.064 | 0.03 |
| MIC50 | 256 | 2 | 0.5 | 1 | 0.25 | 0.5 | 0.12 | 0.12 | 1 | 0.064 | 0.03 |
| MIC90 | 256 | 2 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 2 | 0.125 | 0.03 |
| Range | 32 to 256 | 0.06 to 4 | 0.125 to 2 | 0.03 to 2 | 0.03 to 1 | 0.06 to 8 | 0.015 to 16 | 0.06 to 4 | 0.125 to 32 | 0.023 to 32 | 0.004 to 0.06 |
| Surveillance (97) | |||||||||||
| GM | 246 | 1.66 | 0.58 | 0.69 | 0.20 | 0.33 | 0.14 | 0.17 | 1.25 | 0.19 | 0.02 |
| Mode | 256 | 2 | 1 | 1 | 0.25 | 0.25 | 0.25 | 0.12 | 2 | 0.064 | 0.03 |
| MIC50 | 256 | 2 | 0.5 | 1 | 0.25 | 0.25 | 0.12 | 0.12 | 1 | 0.064 | 0.016 |
| MIC90 | 256 | 2 | 1 | 1 | 0.5 | 1 | 0.25 | 1 | 2 | 0.125 | 0.03 |
| Range | 32 to >256 | 0.12 to 4 | 0.12 to 1 | 0.03 to 2 | 0.03 to 0.5 | 0.06 to 4 | 0.015 to >16 | 0.06 to 4 | 0.25 to 24 | 0.047 to 32 | 0.008 to 0.06 |
| Environmental (17) | |||||||||||
| GM | 256 | 1.17 | 0.43 | 0.54 | 0.14 | 0.19 | 0.07 | 0.10 | 1.54 | 0.06 | 0.03 |
| Mode | 256 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 0.06 | 0.12 | 2 | 0.064 | 0.03 |
| MIC50 | 256 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 0.06 | 0.12 | 2 | 0.064 | 0.03 |
| MIC90 | 256 | 2 | 1 | 1 | 0.25 | 0.5 | 0.12 | 0.12 | 2 | 0.03 | |
| Range | 32 to >256 | 0.12 to 4 | 0.12 to 1 | 0.03 to 2 | 0.03 to 0.5 | 0.06 to 4 | 0.015 to >16 | 0.06 to 4 | 0.25 to 24 | 0.047 to 32 | 0.008 to 0.06 |
TABLE 3.
C. auris MIC distributions for MGX and comparators
| Antifungal | No. of isolates by MIC (mg/liter)a |
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.023 | 0.03 | 0.047 | 0.06 | 0.064 | 0.094 | 0.125 | 0.25 | 0.38 | 0.5 | 0.75 | 1 | 2 | 3 | 4 | 8 | 16 | 24 | 32 | 64 | 128 | ≥256 | |
| MGX | 0 | 0 | 1 | 13 | 77 | 106 | 3 | 0 | 0 | 0 | |||||||||||||||||
| AFG | 0 | 0 | 1 | 9 | 35 | 63 | 71 | 6 | 4 | 10 | 1 | 0 | |||||||||||||||
| CAS | 0 | 6 | 25 | 27 | 61 | 64 | 4 | 2 | 3 | 2 | 0 | 5 | 1b | ||||||||||||||
| MFG | 0 | 0 | 0 | 25 | 130 | 25 | 3 | 3 | 4 | 10 | 0 | ||||||||||||||||
| FLC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 | 194 | |||||||||||||||
| ISA | 0 | 0 | 0 | 2 | 1 | 3 | 5 | 69 | 118 | 2 | 0 | 0 | |||||||||||||||
| ITC | 0 | 0 | 0 | 0 | 2 | 43 | 66 | 88 | 1 | 0 | 0 | 0 | |||||||||||||||
| POS | 0 | 0 | 15 | 26 | 19 | 80 | 59 | 1 | 0 | 0 | 0 | 0 | |||||||||||||||
| VRC | 0 | 0 | 0 | 1 | 1 | 1 | 3 | 44 | 140 | 10 | 0 | 0 | |||||||||||||||
| AMB | 1 | 3 | 3 | 3 | 37 | 60 | 79 | 8 | 2 | 3 | 1 | ||||||||||||||||
| 5FC | 1 | 7 | 23 | 12 | 5 | 6 | |||||||||||||||||||||
Modal MIC values are indicated by underlining and bold font. Shaded values indicate non-wild-type MIC values.
CAS value >16 mg/liter.
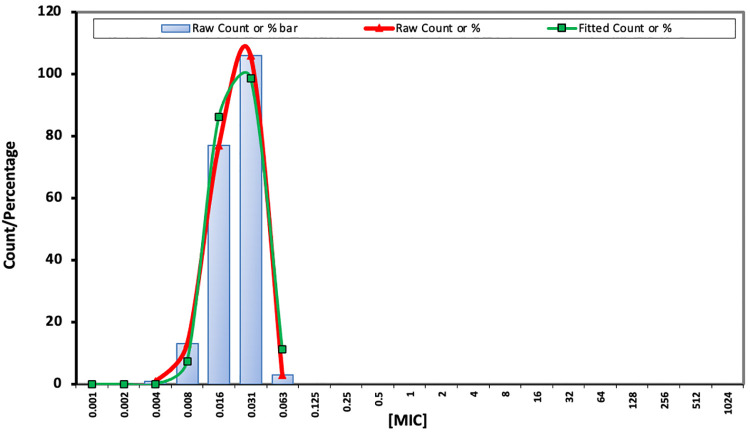
When the subsets of clinical (e.g, blood, abscess, and wound), surveillance (e.g., nares, groin, and axilla), and environmental isolates were evaluated, no differences (within 1- to 2-fold) were found in the MGX or comparator MIC50, MIC90, or GM values (Table 2). MGX demonstrated a narrow distribution range (within five 2-fold serial dilutions) and the MIC50, MIC90, and modal MIC values were 0.03 mg/liter (Table 3). The epidemiologic cutoff value (ECV) 99% for MGX was determined to be 0.06 mg/liter, using the data set of 200 isolates from a single laboratory, as described for ECOFFinder XL 2010 v2.1 (Fig. 1) (27, 28). This local ECV suggests that all isolates were within the population of wild-type (WT) strains and that 0.06 mg/liter defines the upper limit of wild type (UL-WT).
FIG 1.
WT-UL of MGX for NY C. auris isolates was estimated using ECOFFinder.
Limited 5FC susceptibility testing of 54 isolates also demonstrated a narrow MIC range (0.023 to 0.125 mg/liter), with the exception of 6 nonsusceptible isolates demonstrating MIC values of 32 mg/liter (Table 2). The range of values for the echinocandins spanned 9, 11, and 8 2-fold serial dilutions for AFG, CAS, and MFG, respectively (Table 3).
Non-wild-type MIC values were evaluated, based upon CLSI susceptibility breakpoints, CDC recommendations for susceptibility breakpoints, and published C. auris ECVs (AMB, ≥2 mg/liter; AFG, ≥4 mg/liter; CAS, ≥2 mg/liter; and MFG, ≥4 mg/liter)(18, 29) (https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html). A total of 13 C. auris isolates demonstrated MIC values greater than or equal to these values for 1 or more of the echinocandins, with 7 isolates demonstrating evidence of cross-resistance to all 3 echinocandins (see Table S1 in the supplemental material). GM values for these 13 isolates were 3.79 mg/liter (AFG), 4.76 mg/liter (CAS), and 3.06 mg/liter (MFG). Of note, the GM for MGX versus this collection of isolates was 0.013 mg/liter, which is less than the GM for the entire collection of 200 isolates (0.02 mg/liter), consistent with the lack of cross-resistance to the echinocandins.
The MIC values for AMB, as determined by Etest (bioMérieux), ranged from 0.125 to 32 mg/liter; and the GM, mode, MIC50, and MIC90 values were 1.35, 2, 1, and 2 mg/liter, respectively (see Table S2 in the supplemental material). The wide range of values obtained (9 2-fold serial dilutions) suggested non-WT isolates within the collection. Based upon the previously determined C. auris AMB ECV of AMB of ≥2 mg/liter, >50% of the population in this study was non-WT. Using a more stringent cutoff of 3 mg/liter resulted in the identification of 14 isolates (7% of the total study population) with potentially non-WT AMB MIC values, with 6 isolates demonstrating AMB MIC values of ≥16 mg/liter (Table S2). A more stringent cutoff of 3 mg/liter was preferred, as it was obtained from actual testing, as opposed to ≥2 mg/liter, which included isolates with 1.5 mg/liter Etest values rounded off to 2 mg/liter per CDC recommendations (https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html).
None of the echinocandin nonsusceptible isolates (Table S1) were considered AMB resistant, and GM values for AFG, CAS, and MFG were lower for the collection of 14 echinocandin nonsusceptible isolates than the larger collection of 200 isolates. MGX GM MIC values for the total collection of 200 isolates versus the 14 isolates were 0.22 versus 0.26 mg/liter. These data are consistent with a lack of cross-resistance between AMB and the echinocandins as well as AMB and MGX.
The azoles as a group showed a narrower range of MIC values (five to seven 2-fold serial dilutions) than either the echinocandins or AMB (Table 2). All C. auris isolates were FLC resistant using the tentative breakpoint of ≥32 mg/liter. Resistance categories have not been established for C. auris for ISA, ITC, POS, and VRC; however, for C. albicans, isolates are classified as FLC resistant (≥8 mg/liter) or VRC resistant (≥0.5 mg/liter EUCAST), whereas for Candida parapsilosis, VRC-resistant ERG11 mutants were identified with MIC values of ≥1 mg/liter (30–33). Twelve strains were identified with increased azole MIC values, of which 10 strains had VRC MIC values of 4 mg/liter and an additional 2 strains had both ISA and VRC MIC values of 2 mg/liter (see Table S3 in the supplemental material). The WT-UL was determined using EUCAST reference methods and ECOFF finder (27, 34) for C. auris and 95%/99% endpoints were 0.5/1 (ITC), 8/32 (VRC), 1/2 (ISA), and 0.125/0.25 (POS) (18, 28). Using the 99% endpoint values, the data in Table 3 suggest that the numbers of isolates that are non-WT are 1 (ITC), 0 (VRC), 0 (ISA), and 60 (POS). Using the 95% endpoint values, the data in Table 3 suggest that the numbers of isolates that are non-WT are 89 (ITC), 0 (VRC), 2 (ISA), and 140 (POS). Using the rule of thumb that the ECOFF is 2-fold dilutions higher than the mode would suggest that none of the isolates are resistant to any of the azoles, other than FLC.
Five NY C. auris isolates were previously identified as panresistant, based upon the definition of elevated MIC values to FLC, AMB, and the echinocandins (6, 7, 35). The MGX susceptibilities of these isolates, plus that of a sixth isolate obtained in 2020, are shown in Table 4. All six panresistant C. auris isolates were susceptible to MGX (0.008 to 0.016 mg/liter) and had MIC values below the modal value of the larger collection.
TABLE 4.
MGX MIC values for panresistant C. auris
| C. auris strain | MIC (mg/liter) by antifungal agent |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FLC | VRC | ITC | ISA | POS | AFG | CAS | MFG | AMB | 5FC | MGX | |
| 17-24 | 256 | 2 | 0.5 | 0.5 | 0.25 | 4 | >16 | 4 | 2 | 0.064 | 0.016 |
| 18-2 | 256 | 2 | 1 | 1 | 0.25 | 8 | 2 | 4 | 2 | 0.064 | 0.016 |
| 19-42 | 256 | 2 | 1 | 1 | 0.25 | 4 | 16 | 4 | 2 | 0.016 | |
| 19-43 | 256 | 2 | 1 | 1 | 0.25 | 4 | 16 | 4 | 2 | 0.016 | |
| 19-4 | 256 | 2 | 0.5 | 0.5 | 0.25 | 4 | 2 | 4 | 2 | 0.016 | |
| 20-1 | 256 | 2 | 0.5 | 1 | 0.25 | 4 | 4 | 4 | 2 | 0.008 | |
Clade information was available for 62 of the 200 isolates. All were identified as South Asia clade 1, consistent with previous observations that NY C. auris isolates largely belong to South Asia clade I (6).
DISCUSSION
C. auris is an urgent threat in the United States (Antibiotic Resistance Threats in the United States, 2019, https://www.cdc.gov/drugresistance/biggest-threats.html). C. auris isolates involved in the largest U.S. outbreak in the New York metropolitan area are resistant to FLC, variably resistant to other antifungal drugs, and an emergent danger due to observed panresistance (6, 7, 35). C. auris isolates previously recovered in New York were 100% resistant to FLC, 81% resistant to VRC (MIC, ≥2.0 mg/liter), and 61% to AMB (MIC, ≥2.0 mg/liter), with emerging resistance to 1 or more echinocandins (6). The observed pattern is higher than that reported in other published studies, including South Asian clade I isolates (3, 29, 36), and also included organisms with AMB MIC values as high as 32 mg/liter. Active laboratory surveillance of C. auris antifungal resistance patterns remain a high priority. There is an urgent need for the development of new antifungal drugs with different mechanisms of action against C. auris to manage this serious resistance challenge. Additionally, diagnostic laboratories need to expand antifungal test panels to include newly licensed drugs in order to provide evidence-based input for the clinical and public health management of drug-resistant C. auris if drug resistance is a significant problem and new drugs are available in a jurisdiction.
MGX was the most active antifungal agent tested against the 200 C. auris isolates in the present study, and this activity was independent of the clinical, environmental, or surveillance source of C. auris (Table S2). Similarly, no differences in the spectrum of activity of the isolates in the 3 subsets were observed for the comparator drugs, suggesting that the isolates may be derived from common sources. The laboratory-observed pattern of resistance (in vitro testing data) to comparator antifungals did not impact MGX in vitro activity in the present study. The MGX MIC range 0.004 of 0.06 mg/liter was within two dilutions of the ranges reported earlier (16, 17). Additionally, MGX ECV (0.06 mg/liter) in the present study was within two dilutions of the 0.016 mg/liter wild-type upper limit (WT-UL) reported for a collection of 122 Indian C. auris isolates (South Asia clade I) evaluated by the EUCAST methodology (18).
MGX, the active moiety of fosmanogepix (APX001), shows potent activity against various Candida species, except C. krusei, and a wide variety of pathogenic molds (20, 21). The pharmacokinetic (PK)/pharmacodynamic (PD) evaluation of fosmanogepix in the neutropenic mouse model of disseminated candidiasis demonstrated concentration-dependent in vivo efficacy against C. auris and other Candida species (23). In a C. auris disseminated model of infection, mice treated with fosmanogepix demonstrated a higher rate of survival than mice treated with AFG (17). Similarly, in a delayed treatment model utilizing a FLC-resistant strain, fosmanogepix-treated mice demonstrated significant improvements in survival at day 21 as well as reductions in kidney burden (19). The fosmanogepix efficacy observed in animal models awaits further confirmation from ongoing trials for invasive candidiasis/candidemia (37).
The promising profile of MGX includes a unique mode of action and low potential for the development of spontaneous resistance for several Candida species (38). Further examination of the spontaneous mutants of C. albicans and C. parapsilosis by whole-genome sequencing and mutant analysis revealed two efflux-mediated mechanisms, which lead to a 4- to 8-fold increase in MGX MIC values and a concomitant 2- to 4-fold increase in FLC MIC values (39). Previously, a link between elevated MGX values in a subset of FLC-resistant strains was suggested, possibly due to efflux-mediated mechanisms in C. auris as well as other pathogenic yeasts (18, 40). For C. auris, Arendrup et al. (18) reported a wider range of MGX (EUCAST) MIC values (range, 0.001 to 0.125 mg/liter; mode, 0.016 mg/liter) than those observed here. Further analysis showed that isolates with high (>64 mg/liter) FLC MICs had a higher range of MGX MICs (0.004 to 0.06 mg/liter), whereas those with lower FLC MIC values (≤64 mg/liter) demonstrated a lower range of MGX MICs (0.001 to 0.008 mg/liter), with MGX MIC values of 0.004 and 0.008 mg/liter found in both groups. Although elevated MGX MICs were associated with higher FLC MICs, not all of the higher FLC MICs demonstrated elevated MGX MICs, suggesting that some, but not all, FLC resistance mechanisms affect MGX susceptibility (18). In the current study, where all of the isolates had FLC MIC values of ≥256 mg/liter, a narrower range of MGX MICs was observed (0.004 to 0.06 mg/liter), consistent with the range of values observed in the Arendrup et al. study (18). However, although the range of MGX MIC values may be increased 2- to 4-fold in some highly FLC-resistant strains, the MGX MIC values remain low and the clinical impact on the efficacy of fosmanogepix is as yet unknown.
Our observations revealed (1) low MGX MICs against the largest collection of C. auris isolates tested to date; (2) MGX MICs were unchanged against C. auris isolates with variable patterns of resistance to amphotericin B, azoles, and echinocandins; (3) MGX retained potent in vitro activity against six panresistant C. auris isolates, and (4) the CLSI broth microdilution method (41) was reproducible for MGX testing of C. auris. The potent activity of this novel antifungal agent against C. auris supports the continued clinical evaluation for fosmanogepix in the treatment of these often-resistant infections. These findings would also allow us to expand the antifungal susceptibility testing panel to include an MGX evaluation of C. auris from the NY metropolitan area, which might help guide evidence-based management of C. auris clinical and surveillance cases.
MATERIALS AND METHODS
Fungal isolates.
The details of methods used for sample processing for the isolation and characterization C. auris were described recently (6). Two hundred C. auris isolates were selected at random and consisted of 85 clinical, 97 surveillance, and 18 environmental isolates. The details of the various sample types yielding C. auris isolates are summarized in Table S4 in the supplemental material. The numbers of isolates evaluated from each year are as follows: 2 (2017), 58 (2018), 130 (2019), and 10 (2020).
Antifungal susceptibility testing.
The antifungals tested were FLC, VRC, ITC, ISA, POS, AFG, CAS, MFG, AMB, and 5FC. Broth microdilution antifungal susceptibility testing was performed in accordance with Reference Method M27-A3 of the Clinical and Laboratory Standards Institute using TREK frozen broth microdilution panels (catalog number CML2FCAN; Thermo Fisher Scientific, Marietta, OH, USA) for FLC, VRC, ITC, ISA, POS, AFG, CAS, and MFG (41). MICs of AMB for all 200 isolates and 5FC for 54 isolates were determined by Etest at 24 h (AB Biodisk; bioMérieux, Solna, Sweden). 5FC Etest strip availability was limited in the United States, and therefore, we have included results for only 54 isolates. C. albicans ATCC 90028 and C. parapsilosis ATCC 22019, obtained earlier from the American Type Culture Collection, were used as quality-control isolates. The susceptibility breakpoints of the Centers for Disease Control and Prevention were used to assess antifungal resistance patterns in C. auris (FLC, ≥32; AMB, ≥2; AFG, ≥4; CAS, ≥2; and MFG, ≥4 mg/liter), and the Etest AMB MIC value of 1.5 mg/liter was rounded up to 2.0 mg/liter as described (https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html). Susceptibility/resistance to VRC and other triazoles (ITA, ISA, and POS) were assessed using published reports for other Candida species (30–33). We also considered a prior publication on the epidemiological cutoff values of antifungal compounds for 122 C. auris isolates from India (18). As the term is not used conventionally with antifungal drugs, we defined panresistant C. auris as isolates with in vitro resistance to two or more azoles, all echinocandins, and AMB (35).
MGX susceptibility testing.
MGX stock solutions were prepared at 10 mg/ml in 100% dimethyl sulfoxide (DMSO), and aliquots were stored at –20°C. Broth microdilution susceptibility testing was performed according to CLSI M27-A3 guidelines (41). MGX was first diluted in DMSO to obtain intermediate dilutions. These intermediate dilutions were further diluted in microtiter plates to obtain a final concentration of 0.001 to 0.5 mg/liter. A total of 1 μl of DMSO was added to “no drug” control wells. The solutions were mixed on a plate shaker for 10 min, and plates were incubated at 35°C for 24 h. For MGX, the minimum concentration that led to 50% reduction in fungal growth compared with the control was determined as the MIC, as previously described for the echinocandins (41). C. albicans ATCC 90028 and C. parapsilosis ATCC 22019, obtained earlier from the American Type Culture Collection, were used as quality-control (QC) isolates. MGX was tested seven times against two QC strains, and MIC values for C. albicans ATCC 90028 and C. parapsilosis ATCC 22019 were within recommended CLSI ranges (see Table S5 in the supplemental material).
The WT-UL for MGX for MGX were estimated using the Microsoft Excel spreadsheet calculator ECOFFinder (27, 28).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a grant from Amplyx Pharmaceuticals.
M.K. is an employee of Amplyx; K.J.S. was a previous employee of Amplyx and is now an independent consultant. All other authors have no conflicts.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Adams E, Quinn M, Tsay S, Poirot E, Chaturvedi S, Southwick K, Greenko J, Fernandez R, Kallen A, Vallabhaneni S, Haley V, Hutton B, Blog D, Lutterloh E, Zucker H, Candida auris Investigation Workgroup. 2018. Candida auris in Healthcare Facilities, New York, USA, 2013–2017. Emerg Infect Dis 24:1816–1824. doi: 10.3201/eid2410.180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallabhaneni S, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2017. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. Am J Transplant 17:296–299. doi: 10.1111/ajt.14121. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. 2019. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis 25:1780–1781. doi: 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow NA, Muñoz JF, Gade L, Berkow EL, Li X, Welsh RM, Forsberg K, Lockhart SR, Adam R, Alanio A, Alastruey-Izquierdo A, Althawadi S, Araúz AB, Ben-Ami R, Bharat A, Calvo B, Desnos-Ollivier M, Escandón P, Gardam D, Gunturu R, Heath CH, Kurzai O, Martin R, Litvintseva AP, Cuomo CA. 2020. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. mBio 11:e03364-19. doi: 10.1128/mBio.03364-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Y, O’Brien B, Leach L, Clarke A, Bates M, Adams E, Ostrowsky B, Quinn M, Dufort E, Southwick K, Erazo R, Haley VB, Bucher C, Chaturvedi V, Limberger RJ, Blog D, Lutterloh E, Chaturvedi S. 2019. Laboratory analysis of an outbreak of Candida auris in New York from 2016 to 2018: impact and lessons learned. J Clin Microbiol 58:e01503-19. doi: 10.1128/JCM.01503-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrowsky B, Greenko J, Adams E, Quinn M, O'Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S, Lutterloh E, Blog D, C. auris Investigation Work Group. 2020. Candida auris isolates resistant to three classes of antifungal medications—New York, 2019. MMWR Morb Mortal Wkly Rep 69:6–9. doi: 10.15585/mmwr.mm6901a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kordalewska M, Lee A, Park S, Berrio I, Chowdhary A, Zhao Y, Perlin DS. 2018. Understanding echinocandin resistance in the emerging pathogen Candida auris. Antimicrobial Agents Chemotherapy 62:e00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsukahara K, Hata K, Nakamoto K, Sagane K, Watanabe N-A, Kuromitsu J, Kai J, Tsuchiya M, Ohba F, Jigami Y, Yoshimatsu K, Nagasu T. 2003. Medicinal genetics approach towards identifying the molecular target of a novel inhibitor of fungal cell wall assembly. Mol Microbiol 48:1029–1042. doi: 10.1046/j.1365-2958.2003.03481.x. [DOI] [PubMed] [Google Scholar]
- 10.Umemura M, Okamoto M, Nakayama K-i, Sagane K, Tsukahara K, Hata K, Jigami Y. 2003. GWT1 gene is required for inositol acylation of glycosylphosphatidylinositol anchors in yeast. J Biol Chem 278:23639–23647. doi: 10.1074/jbc.M301044200. [DOI] [PubMed] [Google Scholar]
- 11.Hata K, Horii T, Miyazaki M, Watanabe N. 2011. In vitro and in vivo antifungal activities of E1211, a water-soluble prodrug of E1210, abstr F1-1377. Abstr Interscience Conference on Antimicrobial Agents Chemotherapy. American Society for Microbiology, Washington, DC. [Google Scholar]
- 12.Watanabe N-a, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. 2012. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother 56:960–971. doi: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLellan CA, Whitesell L, King OD, Lancaster AK, Mazitschek R, Lindquist S. 2012. Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem Biol 7:1520–1528. doi: 10.1021/cb300235m. [DOI] [PubMed] [Google Scholar]
- 14.Hodges M, Ople E, Shaw K, Mansbach R, Van Marle S, van Hoogdalem E, Kramer W, Wedel P. 2017. Phase 1 study to assess safety, tolerability and pharmacokinetics of single and multiple oral doses of APX001 and to investigate the effect of food on APX001 bioavailability. Open Forum Infect Dis 4:S534. doi: 10.1093/ofid/ofx163.1390. [DOI] [Google Scholar]
- 15.Hodges M, Ople E, Shaw K, Mansbach R, Van Marle S, van Hoogdalem E, Wedel P, Kramer W. 2017. First-in-human study to assess safety, tolerability and pharmacokinetics of APX001 administered by intravenous infusion to healthy subjects. Open Forum Infect Dis 4:S526. doi: 10.1093/ofid/ofx163.1370. [DOI] [Google Scholar]
- 16.Berkow EL, Lockhart SR. 2018. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J Antimicrob Chemother 73:3060–3062. doi: 10.1093/jac/dky302. [DOI] [PubMed] [Google Scholar]
- 17.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendrup MC, Chowdhary A, Astvad KMT, Jørgensen KM. 2018. APX001A in vitro activity against contemporary blood isolates and Candida auris determined by the EUCAST reference method. Antimicrob Agents Chemother 62:e01225-18. doi: 10.1128/AAC.01225-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiederhold NP, Najvar LK, Shaw KJ, Jaramillo R, Patterson H, Olivo M, Catano G, Patterson TF. 2019. Efficacy of delayed therapy with Fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother 63:e01120-19. doi: 10.1128/AAC.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki M, Horii T, Hata K, Watanabe N-A, Nakamoto K, Tanaka K, Shirotori S, Murai N, Inoue S, Matsukura M, Abe S, Yoshimatsu K, Asada M. 2011. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob Agents Chemother 55:4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfaller MA, Hata K, Jones RN, Messer SA, Moet GJ, Castanheira M. 2011. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Candida spp. as determined by CLSI broth microdilution method. Diagn Microbiol Infect Dis 71:167–170. doi: 10.1016/j.diagmicrobio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Pfaller M, Huband M, Flamm R, Bien P, Castanheira M. 2019. In vitro activity of APX001A (Manogepix) and comparator agents against 1,706 fungal isolates collected during an International Surveillance Program in 2017. Antimicrob Agents Chemother 63:e00840-19. doi: 10.1128/AAC.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao M, Lepak AJ, VanScoy B, Bader JC, Marchillo K, Vanhecker J, Ambrose PG, Andes DR. 2018. In vivo pharmacokinetics and pharmacodynamics of APX001 against Candida spp. in a neutropenic disseminated candidiasis mouse model. Antimicrob Agents Chemother 62:e02542-17. doi: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw KJ, Schell WA, Covel J, Duboc G, Giamberardino C, Kapoor M, Moloney M, Soltow QA, Tenor JL, Toffaletti DL, Trzoss M, Webb P, Perfect JR. 2018. In vitro and in vivo evaluation of APX001A/APX001 and other Gwt1 inhibitors against Cryptococcus. Antimicrob Agents Chemother 62:e00523-18. doi: 10.1128/AAC.00523-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viriyakosol S, Kapoor M, Okamoto S, Covel J, Soltow QA, Trzoss M, Shaw KJ, Fierer J. 2018. APX001 and other Gwt1 inhibitor prodrugs are effective in experimental Coccidioides immitis pneumonia. Antimicrob Agents Chemother 63:e01715-18. doi: 10.1128/AAC.01715-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebremariam T, Alkhazraji S, Alqarihi A, Jeon HH, Gu Y, Kapoor M, Shaw KJ, Ibrahim AS. 2018. APX001 is effective in the treatment of murine invasive pulmonary aspergillosis. Antimicrob Agents Chemother 63:e01713-18. doi: 10.1128/AAC.01713-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild‐type MIC value distributions and the determination of epidemiological cut‐off values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 28.Espinel-Ingroff A, Turnidge J. 2016. The role of epidemiological cutoff values (ECVs/ECOFFs) in antifungal susceptibility testing and interpretation for uncommon yeasts and moulds. Rev Iberoam Micol 33:63–75. doi: 10.1016/j.riam.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. 2017. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother 61:e00485-17. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branco J, Silva AP, Silva RM, Silva-Dias A, Pina-Vaz C, Butler G, Rodrigues AG, Miranda IM. 2015. Fluconazole and voriconazole resistance in Candida parapsilosis is conferred by gain-of-function mutations in MRR1 transcription factor gene. Antimicrob Agents Chemother 59:6629–6633. doi: 10.1128/AAC.00842-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricardo E, Grenouillet F, Miranda IM, Silva RM, Eglin G, Devillard N, Rodrigues AG, Pina-Vaz C. 2020. Mechanisms of acquired in vivo and in vitro resistance to voriconazole by Candida krusei following exposure to suboptimal drug concentration. Antimicrob Agents Chemother 64:e01651-19. doi: 10.1128/AAC.01651-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meinike K, Jørgensen KM, Astvad KMT, Hare RK, Arendrup MC. 2019. EUCAST susceptibility testing of isavuconazole: MIC data for contemporary clinical mold and yeast isolates. Antimicrob Agents Chemother 63:e00073-19. doi: 10.1128/AAC.00073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You L, Qian W, Yang Q, Mao L, Zhu L, Huang X, Jin J, Meng H. 2017. ERG11 gene mutations and MDR1 upregulation confer pan-azole resistance in Candida tropicalis causing disseminated candidiasis in an acute lymphoblastic leukemia patient on posaconazole prophylaxis. Antimicrob Agents Chemother 61:e02496-16. doi: 10.1128/AAC.02496-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arendrup MC, Cuenca-Estrella M, Lass-Flörl C, Hope W, EUCAST-AFST. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST)*. Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien B, Chaturvedi S, Chaturvedi V. 2020. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrobial Agents Chemotherapy 64:e02195. doi: 10.1128/AAC.02195-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A, Meis JF, Chowdhary A. 2015. Multidrug-resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J Clin Microbiol 53:1823–1830. doi: 10.1128/JCM.00367-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rauseo AM, Coler-Reilly A, Larson L, Spec A. 2020. Hope on the horizon: novel fungal treatments in development. Open Forum Infect Dis 7:ofaa016. doi: 10.1093/ofid/ofaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapoor M, Moloney M, Soltow QA, Pillar CM, Shaw KJ. 2019. Evaluation of resistance development to the Gwt1 inhibitor manogepix (APX001A) in Candida species. Antimicrob Agents Chemother 64:e01387-19. doi: 10.1128/AAC.01387-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liston SD, Whitesell L, Kapoor M, Shaw KJ, Cowen LE. 2020. Enhanced efflux pump expression in Candida mutants results in decreased manogepix susceptibility. Antimicrob Agents Chemother 64:e00261-20. doi: 10.1128/AAC.00261-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arendrup MC, Jørgensen KM. 2020. Manogepix (APX001A) displays potent in vitro activity against human pathogenic yeast, but with an unexpected correlation to fluconazole MICs. Antimicrob Agents Chemother 64:e00429-20. doi: 10.1128/AAC.00429-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts Approved standard M27-A3. Vol. 28 No. 14 Clinical Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.