Abstract
Transformation is a key step in modern breeding technology that involves genome editing. The requirement for in vitro tissue culture and regeneration hampers application of this technology to commercially important varieties of many crop species. To overcome this problem, we developed a simple and reproducible in planta transformation method in wheat (Tritticum aestivum L.). Our in planta particle bombardment (iPB) method utilizes the shoot apical meristem (SAM) as a target tissue. The SAM contains a subepidermal cell layer termed L2, from which germ cells later develop during floral organogenesis. The iPB method can also be used for genome editing through transient CRISPR/Cas9 expression or direct delivery of the CRISPR/Cas9 ribonucleoprotein. In this review, we describe the iPB technology and provide an overview of its current and future applications in plant transformation and genome editing.
Keywords: biolistic, genome editing, SAM, wheat
Introduction
Genetic transformation is an essential technique for plant biotechnology. Current advancements in crop genome editing using engineered nucleases such as clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) and transcription activator-like effector nucleases (TALENs) have returned transformation to the limelight (Chen et al. 2019; Zhang et al. 2018). However, current strategies for CRISPR/Cas9 application in plants rely mainly on conventional plant transformation procedures that include callus culture and regeneration. This limits the application of transformation technology to genotypes that are amenable to tissue culture (Altpeter et al. 2016; Kausch et al. 2019). Unfortunately, many elite commercial varieties of wheat (Tritticum aestivum L.) and barley (Hordeum vulgare L.) are recalcitrant to transformation or genome editing. In addition, callus culture is generally time-consuming and can be affected by somatic variation. Development of a novel plant transformation method that is independent of tissue culture and regeneration is necessary to avoid issues associated with these processes.
In planta transformation
In planta transformation involves the transformation of intact plants or plant tissues without callus culture or regeneration. The most well-known and widely used in planta transformation method is the floral dip method developed for Arabidopsis thaliana (Clough and Bent 1998). In this method, flower buds are dipped into Agrobacterium suspension or Agrobacterium inoculant is dropped onto buds to provide contact between germ cells and Agrobacterium. Seeds from these flowers are transformed at a low but consistent rate. The mechanism of floral dip transformation is based on the amenability of ovule cells to Agrobacterium infection (Bent 2000). Currently, Arabidopsis transformation is performed exclusively using the floral-dip method. Although this simple and cost-effective protocol seems attractive to researchers of other plant species, application to species other than Arabidopsis is not so simple. Floral dip methods have been reported for rice (Ratanasut et al. 2017), wheat (Zale et al. 2009), radish (Curtis and Nam 2001), and Medicago truncatula (Trieu et al. 2000). However, successful application of these protocols in other laboratories appears lacking, suggesting difficulty in reproducing the transformation conditions. Direct delivery of DNA into pollen cells has also been described. Transgenic maize plants were created by using ultrasonication to facilitate DNA introduction into pollen cells (Yang et al. 2017). Magnetofection was applied to cotton pollen to create transgenic plants (Zhao et al. 2017).
In addition to floral organs, the shoot apical meristem (SAM) represents a suitable target for in planta transformation. McCabe and co-workers successfully created fertile transgenic soybean (McCabe et al., 1988) and cotton (McCabe and Martinell 1993) by particle bombardment followed by in situ multiple shoot induction. However, the efficiency of heritable transformation was low, and addition of herbicide resistance selection may be necessary (Aragão et al. 2000). Agrobacterium-mediated transformation of the SAM by piercing the meristematic tissue of seed embryos has been used to create transgenic rice (Supartana et al. 2005) and wheat plants (Supartana et al. 2006). When tested in our laboratory, the efficiency of obtaining transgenic wheat plants using seed embryonic SAMs was low and inconsistent. Overall, however, these studies indicate that germline transformation using the SAM is possible.
In planta particle bombardment (iPB)
We recently developed a transformation protocol for wheat, in planta particle bombardment (iPB), which targets the seed embryonic SAM (Figure 1) (Hamada et al., 2017). The iPB method does not require callus culture or regeneration and is thus applicable for a variety of commercial varieties. The standard iPB procedure is summarized below (Hamada et al. 2017).
Figure 1. Outline of the iPB method for transformation and genome editing. See text for description of the procedure.

Step 1. Preparation of material
The SAM is usually located in the center of the shoot apical region and covered with several layers of newly developed leaves. SAM isolation can be very difficult because these leaf tissues have to be removed carefully. There is some merit to using seed embryos because few leaves are developed; dry mature wheat seeds contain three leaves. To obtain SAM tissue suitable for the iPB method, it is necessary to remove the coleoptile and the first three leaves from imbibed seed embryos under a stereo microscope (Figure 1). This can be achieved using an insulin pen needle (34 G). Embryos are then isolated from the seeds and arranged circularly on an agar plate. It takes 3 min or less for a well-trained operator to process one seed.
Step 2. Preparation of particles
Gold particles (diameters 0.3–1.2 µm) are used for iPB transformation. Since physical properties of cells differ substantially in different tissues and species, gold particle size should be optimized for each plant material. Coating of DNA onto the surface of gold particles is achieved by ethanol precipitation (Hamada et al. 2017).
Step 3. Particle bombardment
We routinely use a Biorad PDS-1000/He for bombardment, but alternative particle delivery systems should also work. Helium gas pressures of 1,100 or 1,350 psi are typically used. Three or four shots are delivered per plate of embryos. When a GFP gene expression cassette is introduced, 60–90% of bombarded embryos show fluorescent signals in the SAM (Figure 1).
Step 4. Screening for positive clones
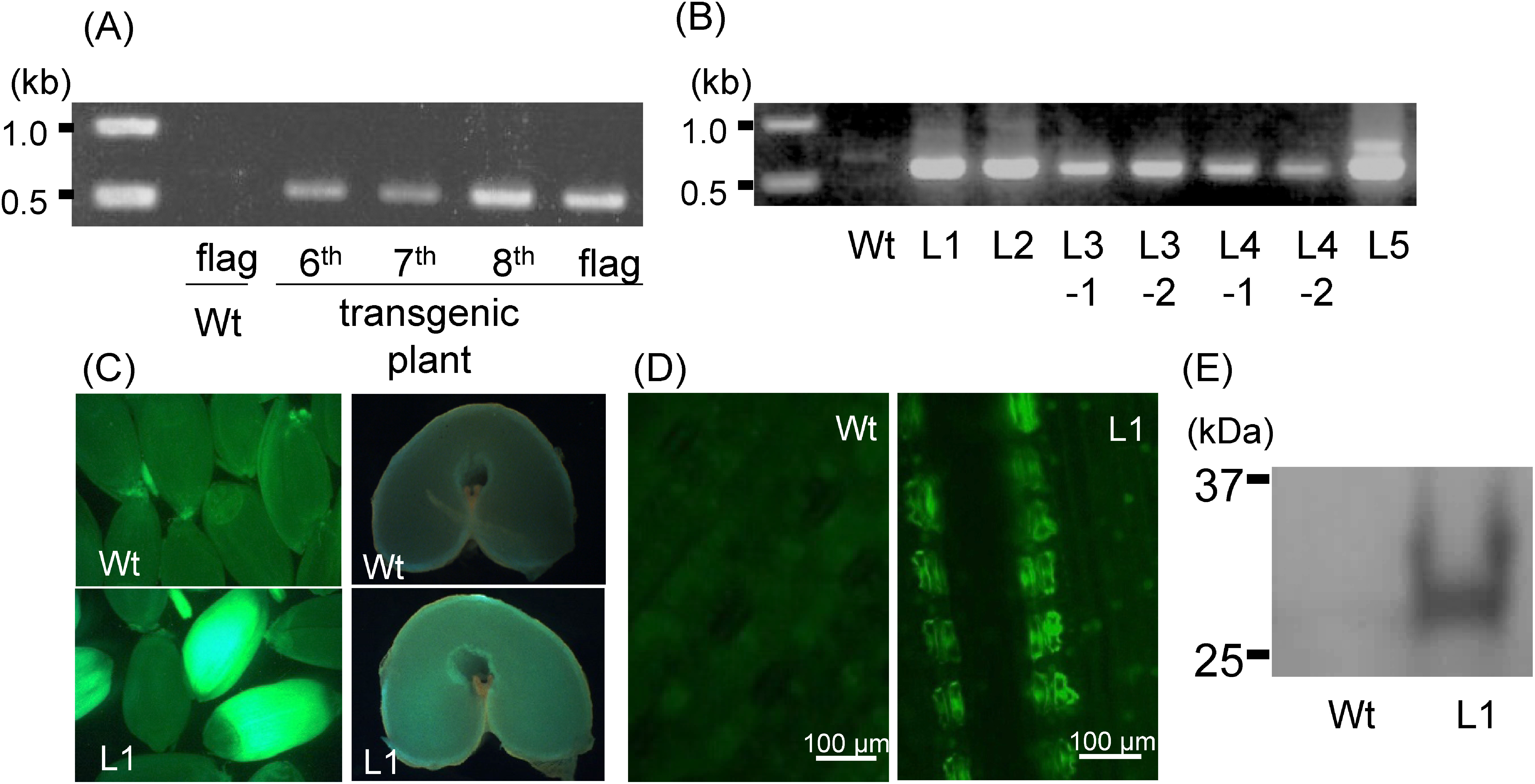
If SAMs are exposed properly, most bombarded embryos will grow normally. Plants can be transferred to soil when shoots and roots are established. Screening for candidate transformants is performed by genomic PCR (Figure 1). Transformation efficiency is high enough that antibiotic selection is not necessary. Since the first to the third leaves are already developed prior to bombardment, tissue from the fifth or later leaves may be analyzed by genomic PCR to detect stable transformation (Figure 2A).
Figure 2. Analysis of transgenic plants. A. Genomic PCR analysis of a putative transgenic ‘Fielder’ line and wild-type (Wt) line. Genomic DNA was extracted from the indicated leaves of a putative T0 wheat plant. B. PCR analysis of five independent transgenic T1 (L1–L5) and wild-type (Wt) ‘Fielder’ lines. Genomic DNA was extracted from the first leaf of each T1 progeny. C. GFP accumulation in T1 seeds of the L1 transgenic plant. Left: fluorescence image of whole Wt (upper) and L1 (lower) seeds. Right: fluorescence images of sections of Wt (upper) and L1 (lower) seeds. D. GFP image of T1 leaf. Young leaves of L1 (T1 progeny) and wild-type (Wt) plants. E. Total protein from transgenic (L1) and Wt plants. Fluorescent images captured by FUJIFILM LAS3000 equipped with a GFP filter (Ex: 460, Em: 510).
Step 5. Analysis of T1 plants
With the iPB method, candidate transgenic plants in the T0 generation are chimeric, containing transgenic and non-transgenic cells. It is therefore necessary to select heritable transgenic plants at the T1 generation (Figure 2B). Following transformation with a GFP construct, 31–83% of T1 progeny inherited the transgene (Hamada et al. 2017). Transgene integration is analyzed in T1 plants. Generally, multicopy integration is observed; however, transgene copy numbers are relatively low compared with conventional culture-based methods (Hamada et al. 2017). This may be because the iPB method does not use antibiotic selection, which may preferentially select high copy number clones. In the T1 generation, GFP gene expression is detected in seeds and leaf tissue (Figure 2C–E). Absence of transgene expression may result from multicopy silencing or lack of full construct integration. Transformation efficiency using the iPB method is 0.17–0.35%, and there seems to be no genotype dependency (Hamada et al. 2017). With the iPB method, Japanese elite varieties such as ‘Haruyokoi’ and ‘Yumechikara,’ which are recalcitrant to conventional transformation methods, are now amenable to transformation.
Step 6. Mechanism of transformation based on the iPB method
The SAM is composed of three cell layers (L1–L3) dividing independently (Figure 3). The epidermal (L1) and subepidermal (L2) layers are single cell layers that divide anticlinal to the plane. The corpus (L3) cell layer constitutes the remainder of the SAM and exhibits both anticlinal and periclinal division. The three layers have distinct fates during floral organogenesis. Classical studies with periclinal chimeras revealed that L1 forms epidermis while L2 differentiates into germ cells such as pollen and embryo sac (Goldberg et al. 1993; Reiser and Fischer 1993). Genome modifications occurring in L2 cells of the SAM can be passed to the next generation via germ cell development. Therefore, L2 cells of the SAM are the targets in the iPB method (Figure 3).
Figure 3. Principle of the iPB method. Gold nanoparticles can bind DNA, RNA, and protein and deliver these materials into L2 layer cells, which develop into the pollen and embryo sac.

Merits of the iPB method
There are several merits to using the iPB method over culture-based transformation methods. The iPB method does not require callus culture or regeneration, making it applicable to cultivars that are recalcitrant to conventional transformation methods. Transformants are selected by genomic PCR screening; no antibiotic selection is necessary. Circumvention of tissue culture-based antibiotic selection greatly accelerates the production of transgenic plants.
The iPB method for genome editing
The successful wheat transformation via iPB suggests it as a possible method for genome editing. It is becoming clear that constitutive expression of CRISPR/Cas9 is unnecessary and that the short-term presence of CRISPR/Cas9 in cells is sufficient to produce editing (Woo et al. 2015; Zhang et al. 2016). This can be achieved by transient expression of CRISPR/Cas9. We estimate the efficiency of transient transformation using iPB to be much higher than that of stable transformation (0.17–0.35%). We therefore introduced genes for SpCas9 and guide RNA into SAM for transient expression (Hamada et al. 2018). Gold particles coated with plasmids expressing CRISPR/Cas9 components designed to target the TaLOX2 gene (Zhang et al. 2016) were bombarded into SAM-exposed embryos of imbibed mature wheat seeds. Mutations in the target gene were assessed in fifth-leaf tissue using cleaved amplified polymorphic sequence (CAPS) analysis (Figure 4A). Eight of 210 bombarded plants (3.8%) carried mutant alleles. Candidate T0 plants (LX1–LX8) were harvested and T1 plants were subjected to CAPS assay. One of the T0 plants, LX4, passed the mutation to the next generation (T1). Cas9 and guide RNA sequences designed to target TaGASR7 were also bombarded into SAM-exposed embryos of imbibed mature seeds (Hamada et al., 2018). Mutations in the target sequence were assessed by fifth-leaf cleaved amplified polymorphic sequence (CAPS) analysis. Eleven of 210 bombarded plants (5.2%) carried mutant alleles, and the mutations of three (1.4%) of these were inherited by the next generation (T1). Genotype analysis of T1 plants identified plants homozygous for the edited gene. These plants showed no detectable integration of the Cas9 and guide RNA sequences, indicating that the mutations were introduced through transient expression of CRISPR/Cas9 (Hamada et al. 2018).
Figure 4. Genome editing using the iPB-DNA method. A. CAPS assay of TaLOX2 candidate edited plants. The TaLOX2 target region was amplified by PCR from total DNA isolated from the fifth leaf of T0 plants, digested with SacI and separated on an agarose gel. B. Genome editing efficiency of TaLOX2 and TaGASR7.

The iPB method for DNA-free editing
In addition to DNA, gold particles can bind RNA and proteins and successfully introduce these into plant cells through particle bombardment (Davis et al. 1999; Martin-Ortigosa and Wang 2014). This property of gold particles is attractive for genome editing. There are several reports that direct introduction of CRISPR/Cas9 ribonucleoproteins (RNPs) into cells can efficiently create genome-edited plants (Liang et al. 2017; Svitashev et al. 2016; Woo et al. 2015). We examined whether the iPB method can directly introduce CRISPR/Cas9 RNP into SAMs for successful genome editing. By optimizing conditions for the gold–RNP complex and microcarrier preparation and binding, we obtained genome-edited T1 plants. The efficiency of obtaining genome-edited T1 plants, calculated for three target genes, was 0.9–4.2% (mean: 3.0%), comparable to that of the iPB method with CRISPR/Cas9 DNA.
Summary and future directions
Here, we have reviewed the current status of the iPB method for plant biotechnology. The iPB method is one solution to the problem of genotype dependency of wheat transformation and genome editing. Elite cultivars, either spring type or winter type, are now amenable to genome editing. Future application of the iPB method may take several directions. First, this technique may be applicable to many monocot and dicot plants. Those whose SAM is easily isolated and able to grow normally after isolation are good candidates. Crops for which mericlone propagation is available are also possible targets. Second, protein delivery is possible using this method. Introduction of TALENs to the SAM may allow non-transgenic genome editing. It may also be possible to change the fate of the SAM by delivering proteins important for SAM function. Third, genome editing components together with template DNA can be introduced to the SAM to attain genome editing using homologous recombination. The iPB method and its application are therefore expected to contribute to the development of plant biotechnology and genome editing.
Acknowledgments
We thank Drs. Masaki Endo, Fumitaka Abe and Seiichi Toki for providing vectors. We are grateful to Drs Kentaro Sasaki, Etsuo Shimosaka, Midori Yoshida, and Takayuki Saito for suggestions and comments. We also would like to thank our group members at Hokkaido Agricultural Research Center (HARC) and Institute of Agrobiological Sciences (NIAS) for their support and input. This work was supported in part by Cabinet Office, Government of Japan, Cross-ministerial Strategic Innovation Promotion Program (SIP), “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO).
Abbreviations
- CRISPR
clustered regularly interspaced short palindromic repeats
- TALENs
transcription activator-like effector nucleases
- SAM
shoot apical meristem
- iPB
in planta particle bombardment
- RNPs
ribonucleoproteins
- CAPS
cleaved amplified polymorphic sequence
References
- Altpeter F, Springer NM, Bartley LE, Blechl AE, Brutnell TP, Citovsky V, Conrad LJ, Gelvin SB, Jackson DP, Kausch AP, et al. (2016) Advancing crop transformation in the era of genome editing. Plant Cell 28: 1510–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragão FJL, Sarokin L, Vianna GR, Rech EL (2000) Selection of transgenic meristematic cells utilizing a herbicidal molecule results in the recovery of fertile transgenic soybean [Glycine max (L.) Merril] plants at a high frequency. Theor Appl Genet 101: 1–6 [Google Scholar]
- Bent AF (2000) Arabidopsis in planta transformation: Uses, mechanisms, and prospects for transformation of other species. Plant Physiol 124: 1540–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Wang Y, Zhang R, Zhang H, Gao C (2019) CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu Rev Plant Biol 70: 667–697 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Curtis IS, Nam HG (2001) Transgenic radish (Raphanus sativus L. longipinnatus Bailey) by floral-dip method: Plant development and surfactant are important in optimizing transformation efficiency. Transgenic Res 10: 363–371 [DOI] [PubMed] [Google Scholar]
- Davis RE, Parra A, LoVerde PT, Ribeiro E, Glorioso G, Hodgson S (1999) Transient expression of DNA and RNA in parasitic helminths by using particle bombardment. Proc Natl Acad Sci USA 96: 8687–8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RB, Beals TP, Sanders PM (1993) Anther development: Basic principles and practical applications. Plant Cell 5: 1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Linghu Q, Nagira Y, Miki R, Taoka N, Imai R (2017) An in planta biolistic method for stable wheat transformation. Sci Rep 7: 11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Liu Y, Nagira Y, Miki R, Taoka N, Imai R (2018) Biolistic-delivery-based transient CRISPR/Cas9 expression enables in planta genome editing in wheat. Sci Rep 8: 14422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausch AP, Nelson-Vasilchik K, Hague J, Mookkan M, Quemada H, Dellaporta S, Fragoso C, Zhang ZJ (2019) Edit at will: Genotype independent plant transformation in the era of advanced genomics and genome editing. Plant Sci 281: 186–205 [DOI] [PubMed] [Google Scholar]
- Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, Liu J, Zhang H, Liu C, Ran Y, et al. (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8: 14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ortigosa S, Wang K (2014) Proteolistics: A biolistic method for intracellular delivery of proteins. Transgenic Res 23: 743–756 [DOI] [PubMed] [Google Scholar]
- McCabe DE, Martinell BJ (1993) Transformation of elite cotton cultivars via particle bombardment of meristems. Nat Biotechnol 11: 596–598 [Google Scholar]
- McCabe DE, Swain WF, Martinell BJ, Christou P (1988) Stable transformation of soybean (Glycine Max) by particle acceleration. Nat Biotechnol 6: 923–926 [Google Scholar]
- Ratanasut K, Rod-In W, Sujipuli K (2017) In planta Agrobacterium-Mediated transformation of rice. Rice Sci 24: 181–186 [Google Scholar]
- Reiser L, Fischer RL (1993) The ovule and the embryo sac. Plant Cell 5: 1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supartana P, Shimizu T, Nogawa M, Shioiri H, Nakajima T, Haramoto N, Nozue M, Kojima M (2006) Development of simple and efficient in planta transformation method for wheat (Triticum aestivum L.) using Agrobacterium tumefaciens. J Biosci Bioeng 102: 162–170 [DOI] [PubMed] [Google Scholar]
- Supartana P, Shimizu T, Shioiri H, Nogawa M, Nozue M, Kojima M (2005) Development of simple and efficient in planta transformation method for rice (Oryza sativa L.) using Agrobacterium tumefaciens. J Biosci Bioeng 100: 391–397 [DOI] [PubMed] [Google Scholar]
- Svitashev S, Schwartz C, Lenderts B, Young JK, Mark Cigan A (2016) Genome editing in maize directed by CRISPR–Cas9 ribonucleoprotein complexes. Nat Commun 7: 13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu AT, Burleigh SH, Kardailsky IV, Maldonado-Mendoza IE, Versaw WK, Blaylock LA, Shin H, Chiou TJ, Katagi H, Dewbre GR, et al. (2000) Transformation of Medicago truncatula via infiltration of seedlings or flowering plants with Agrobacterium. Plant J 22: 531–541 [DOI] [PubMed] [Google Scholar]
- Woo JW, Kim J, Kwon S, Corvalán C, Cho SW, Kim H, Kim SG, Kim ST, Choe S, Kim JS (2015) DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat Biotechnol 33: 1162–1164 [DOI] [PubMed] [Google Scholar]
- Yang L, Cui G, Wang Y, Hao Y, Du J, Zhang H, Wang C, Zhang H, Wu SB, Sun Y (2017) Expression of foreign genes demonstrates the effectiveness of pollen-mediated transformation in Zea mays. Front Plant Sci 8: 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale JM, Agarwal S, Loar S, Steber CM (2009) Evidence for stable transformation of wheat by floral dip in Agrobacterium tumefaciens. Plant Cell Rep 28: 903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, Qiu J-L, Gao C (2016) Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat Commun 7: 12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Massel K, Godwin ID, Gao C (2018) Applications and potential of genome editing in crop improvement. Genome Biol 19: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Meng Z, Wang Y, Chen W, Sun C, Cui B, Cui J, Yu M, Zeng Z, Guo S, et al. (2017) Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat Plants 3: 956–964 [DOI] [PubMed] [Google Scholar]


