Abstract
Objective:
Although an association between exposure to alcohol advertising and underage drinking is well documented, the underlying neurobiological contributions to this association remain largely unexplored. From an epidemiological perspective, identifying the neurobiological plausibility of this exposure–outcome association is a crucial step toward establishing marketing as a contributor to youth drinking and informing public policy interventions to decrease this influence.
Method:
We conducted a critical review of the literature on neurobiological risk factors and adolescent brain development, social influences on drinking, and neural contributions to reward sensitization and risk taking. By drawing from these separate areas of research, we propose a unified, neurobiological model of alcohol marketing effects on underage drinking.
Results:
We discuss and extend the literature to suggest that responses in prefrontal–reward circuitry help establish alcohol advertisements as reward-predictive cues that may reinforce consumption upon exposure. We focus on adolescence as a sensitive window of development during which youth are particularly susceptible to social and reward cues, which are defining characteristics of many alcohol advertisements. As a result, alcohol marketing may promote positive associations early in life that motivate social drinking, and corresponding neurobiological changes may contribute to later patterns of alcohol abuse.
Conclusions:
The neurobiological model proposed here, which considers neurodevelopmental risk factors, social influences, and reward sensitization to alcohol cues, suggests that exposure to alcohol marketing could plausibly influence underage drinking by sensitizing prefrontal–reward circuitry.
Epidemiological research has established an association between alcohol advertisement exposure and alcohol consumption in youth (Anderson et al., 2009; Hastings et al., 2005; McClure et al., 2013; Smith & Foxcroft, 2009; Snyder et al., 2006). However, the biological plausibility of this association remains unclear. Elucidating the biological underpinnings of this association would not only contribute to an understanding of how real-world alcohol cues (i.e., advertisements) might influence drinking behavior in youth but also would improve policy recommendations and early prevention strategies by considering neurobiological vulnerabilities in the developing brain. For example, by testing competing hypotheses and identifying specific vulnerabilities associated with adolescent brain development, policymakers would be better informed to make decisions about regulations on alcohol marketing strategies that might directly or indirectly target adolescents.
Despite the potential scientific and policy-level advantages, research investigating the impact of alcohol marketing on the developing brain is sparse. In contrast to a systematic review that aims to comprehensively synthesize an existing body of work, here we leverage a narrative approach to contextualize recent work on neural responses to alcohol advertising within the broader literature. The goal of this review is to strengthen the motivation for investigating relationships between neural responses to alcohol advertising and underage drinking by highlighting work directly testing this association and by discussing relevant literature and hypotheses that may inform future work. Following general guidelines for a narrative overview (Green et al., 2006), we first identified published studies that examine neural responses to alcohol advertisements and subsequently expanded this selection to incorporate related literature. To bridge previous work and suggest potential relationships, we propose a neurobiologically informed model (Figure 1) that extends established work on addiction (Koob & Volkow, 2010; Noël et al., 2006) and adolescent brain development (Casey, 2015). More specifically, we discuss neurodevelopmental influences on sensitivity to socioenvironmental cues such as advertisements, as well as adolescent risk taking. We suggest that early drinking (among other risky behaviors) is influenced by hierarchical changes in developing neural circuitry that occur during adolescence, and that an imbalance in the relative maturation of brain circuits may modulate susceptibility to advertisements and risky behaviors associated with drinking (Casey et al., 2019). In addition, social motivation and peer influence exacerbate cue sensitivity during adolescence and reinforce drinking behaviors via the rewarding properties of the action itself (e.g., drinking and pleasure) and conformity to social norms of peer groups. Last, we highlight a process of reinforcement that may motivate continued consumption by sensitizing to social norms of peer groups. Last, we highlight a process of reinforcement that may motivate continued consumption by sensitizing reward circuitry and interfering with the normative development of cognitive control circuitry.
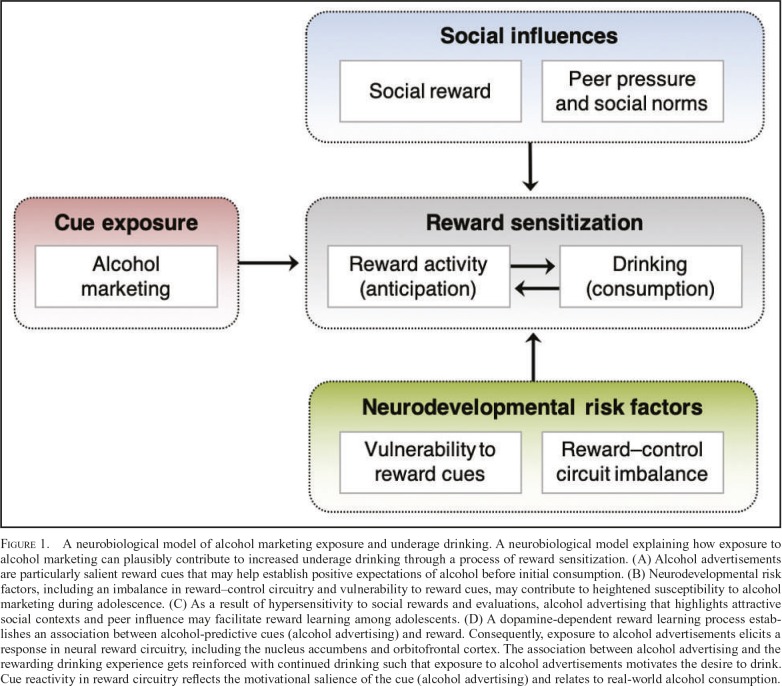
Figure 1.
A neurobiological model of alcohol marketing exposure and underage drinking. A neurobiological model explaining how exposure to alcohol marketing can plausibly contribute to increased underage drinking through a process of reward sensitization. (A) Alcohol advertisements are particularly salient reward cues that may help establish positive expectations of alcohol before initial consumption. (B) Neurodevelopmental risk factors, including an imbalance in reward–control circuitry and vulnerability to reward cues, may contribute to heightened susceptibility to alcohol marketing during adolescence. (C) As a result of hypersensitivity to social rewards and evaluations, alcohol advertising that highlights attractive social contexts and peer influence may facilitate reward learning among adolescents. (D) A dopamine-dependent reward learning process establishes an association between alcohol-predictive cues (alcohol advertising) and reward. Consequently, exposure to alcohol advertisements elicits a response in neural reward circuitry, including the nucleus accumbens and orbitofrontal cortex. The association between alcohol advertising and the rewarding drinking experience gets reinforced with continued drinking such that exposure to alcohol advertisements motivates the desire to drink. Cue reactivity in reward circuitry reflects the motivational salience of the cue (alcohol advertising) and relates to real-world alcohol consumption.
I. Alcohol Advertisements as Reward Cues
The brain’s reward circuitry comprises a network of regions that are sensitive to cues that have been associated with subjective feelings of pleasure. These reward–cue associations have been characterized by the transmission of dopamine (Wise & Rompre, 1989) originating in the midbrain and extending rostrally toward the ventral striatum and orbitofrontal cortex (OFC). Seminal work conducted in animals has demonstrated an increase in dopaminergic firing rates in response to cues that predict a reward, even when the reward itself is not received (Schultz et al., 1997). By learning about and predicting the values of potential rewards, dopamine neurons help motivate and guide adaptive behavior in the real world.
Recent advances in human neuroimaging have allowed researchers to capitalize on this observation by measuring brain activity in response to reward cues. For example, functional magnetic resonance imaging studies have used cue-reactivity paradigms to explore the neural correlates of reward processes by presenting a variety of reward-predictive cues (e.g., images of food, alcohol, other drugs) to participants in the scanner. These studies have consistently demonstrated increased activity in the OFC and ventral striatum, which includes the nucleus accumbens (NAcc), in response to cues across various reward domains (e.g., alcohol cues [Courtney et al., 2018c], cigarette cues [David et al., 2005; Wagner et al., 2011], food cues [Demos et al., 2012; Rapuano et al., 2016; van der Laan et al., 2011], and drug cues [Tang et al., 2012; Wilson et al., 2004]), implicating this network of dopamine-rich regions in processing information about reward in humans.
Although the engagement of NAcc–OFC circuitry has been implicated across reward domains, the magnitude of activity within this circuitry has been shown to vary widely across individuals. Because the reward system is responsible for attaching motivational salience to reward-predictive cues (Berridge & Robinson, 1998), this variability has been observed to reflect individual differences in consumption-related desires and behaviors (Kühn & Gallinat, 2011; Lopez et al., 2014; Robinson & Berridge, 1993). For example, reward-related responses to images of food cues are stronger for obese compared with lean individuals (Bruce et al., 2010; Rapuano et al., 2016; Stoeckel et al., 2008), responses to cigarette cues are greater for smokers relative to nonsmokers (Wagner et al., 2011), and responses to sexual scenes are stronger for sexually active young adults than for those who are not sexually active (Demos et al., 2012). Of particular relevance to the current review, a recent meta-analysis of the literature suggests that alcohol cues reliably activate reward-related brain regions in adults with alcohol use disorders and that activity in these regions—in particular, the ventral striatum and OFC—is associated with alcohol use metrics such as alcohol use severity, self-reported alcohol consumption, and in-scanner craving (Schacht et al., 2013).
Marketing strategies influence responses to advertisements
Recent work has begun to explore neural responses to advertisements for unhealthy products, with the goal of understanding their relation to real-world consumption. For example, studies examining neural responses to alcohol advertisements (Courtney et al., 2018c) and fast-food commercials (Gearhardt et al., 2014; Rapuano et al., 2016, 2017) observed heightened activity in brain regions associated with processing rewards (e.g., NAcc, OFC). Crucially, the variability in response to advertisements within these regions correlated with respective consumption behaviors and health-related outcomes, such that responses to alcohol advertisements were associated with real-world drinking behavior (Figure 2; Courtney et al., 2018c) and responses to food commercials were associated with obesity metrics (Rapuano et al., 2016). These findings suggest that responses to advertisements within these regions may be a meaningful predictor of real-world outcomes.
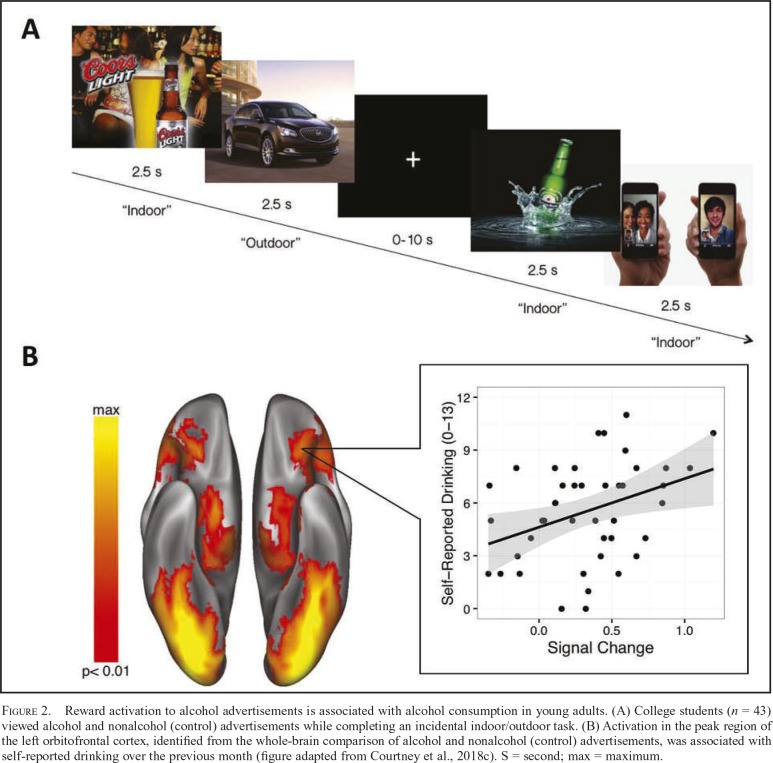
Figure 2.
Reward activation to alcohol advertisements is associated with alcohol consumption in young adults. (A) College students (n = 43) viewed alcohol and nonalcohol (control) advertisements while completing an incidental indoor/outdoor task. (B) Activation in the peak region of the left orbitofrontal cortex, identified from the whole-brain comparison of alcohol and nonalcohol (control) advertisements, was associated with self-reported drinking over the previous month (figure adapted from Courtney et al., 2018c). S = second; max = maximum.
Although previous studies have applied similar “brain-as-predictor” methods to explore real-world outcomes (Berkman & Falk, 2013) using well-controlled, standardized stimuli (e.g., unlabeled static images of food; Demos et al., 2012; Lopez et al., 2014), advertisements provide a unique opportunity to explore neural responses to ecologically valid real-world cues. Marketing strategies influence the way in which products are perceived and experienced and may correspondingly alter reward responses to these products. For example, relative to consuming wine labeled with a low price tag, consuming the same wine with a high price tag is perceived as more enjoyable and produces heightened activity in the OFC (Plassmann et al., 2008). Similarly, obese individuals demonstrate heightened neural activity for milkshakes labeled as high fat relative to low fat, even when the milkshakes contain an equal calorie content (Ng et al., 2011). Conversely, food images labeled with calorie information (relative to unlabeled food images) elicit heightened activity in control-related circuitry and attenuated activity in reward circuitry (Courtney et al., 2018a), further underscoring the idea that neural responses to advertised products are sensitive to framing and the consumer’s expectations.
Another way that marketing may influence responses to advertisements is through repeated pairing of brand logos (or slogans) with rewarding contexts or outcomes, which may be unrelated to the advertised product. That is, a conditioned stimulus (e.g., an arbitrary symbol or aluminum beer can) can become endowed with positive expectations through mere exposure. Because exposure to food and alcohol advertising begins in early childhood, these positive associations are evident early in development and can occur before consumption or substance use. For example, a study of fifth- and sixth-grade nondrinkers showed a relationship between awareness of beer advertising and favorable beliefs about drinking, as well as an increased intention to drink in the future (Grube & Wallack, 1994). In addition, rhesus macaques develop pseudo-“brand preferences” simply by pairing logos (e.g., for Pizza Hut) with social and sexual cues (Acikalin et al., 2018). These studies demonstrate a bias for marketed products or brands based on positive associations with socioenvironmental cues, independent of consumptive experience.
The alcohol industry alleges that marketing efforts direct consumer attention toward particular brands but do not encourage more drinking overall (Beer Institute, 2015). However, recent research has demonstrated that attaching an in-group affiliation (e.g., a university logo) to alcohol branding increases the motivational salience of the beverage among college students (Bartholow et al., 2018), and drinkers who report strong alcohol brand preferences tend to binge drink more than those without brand preferences (Tanski et al., 2011). Further, human neuroimaging studies have observed increased activity in reward-related circuitry in response to fast-food logos in healthy-weight children (Bruce et al., 2014) as well as decreased activity in cognitive control circuitry in obese children (Bruce et al., 2013), suggesting that consumptive experiences with marketed products influence future responses to brands. As a consequence, advertisements that tap into idiosyncratic brand preferences and facilitate the development of consumer loyalty may engender a stronger pull on drinking behavior.
Despite the recent increase in neuroimaging studies aimed at understanding neural responses to advertisements, no study has yet directly compared responses between advertisements and standardized cue-reactivity tasks. To characterize brain activity associated with marketing strategies in the context of the broader literature, an important next step will be to formally compare a naturalistic paradigm using advertisements with a well-controlled paradigm using validated reward cues within the same participants.
II. Neurodevelopmental Vulnerability
Previous studies have pointed to a strong inverse association between age at first drink and later alcohol abuse (Grant & Dawson, 1997; Hawkins et al., 1997; Prescott & Kendler, 1999; Zeigler et al., 2005), suggesting that the initiation of alcohol consumption may be a meaningful predictor of problematic drinking later in life. However, the neurodevelopmental factors that contribute to vulnerability to early alcohol consumption remain unclear. Here we highlight adolescence as a sensitive developmental window during which reward circuitry is particularly responsive to environmental cues such as advertisements, and we further discuss how an imbalance in developing neural circuitry may influence adolescent risk taking.
Adolescence is characterized as a developmental window during which substantial physical, emotional, and cognitive changes occur. Importantly, these changes are coupled with significant changes in the brain (Blakemore & Choudhury, 2006; Casey, 2015). Brain regions involved in processing rewards and appetitive cues (e.g., NAcc) show structural (Mills et al., 2014), functional (Galvan et al., 2006), and neurochemical maturational changes (Brenhouse & Andersen, 2011) early in adolescence, whereas brain circuitry involved in the regulation of reward impulses (e.g., the prefrontal cortex [PFC]; Somerville et al., 2011) shows more protracted changes with development (Figure 3). Without a commensurate increase in the ability to exert regulatory control, the early maturation of reward circuitry leads to a functional imbalance wherein adolescent reward circuitry becomes hypersensitive to appetitive cues (Casey, 2015; Somerville & Casey, 2010).
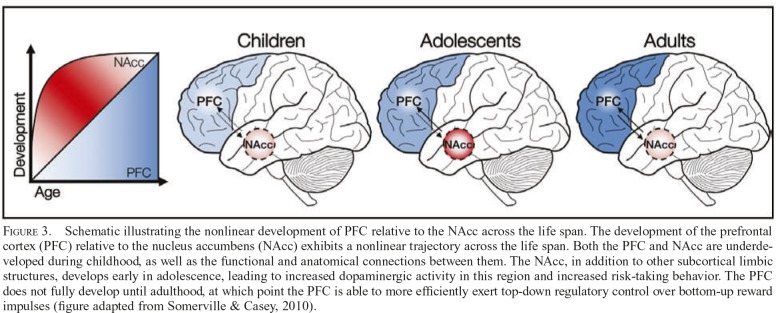
Figure 3.
Schematic illustrating the nonlinear development of PFC relative to the NAcc across the life span. The development of the prefrontal cortex (PFC) relative to the nucleus accumbens (NAcc) exhibits a nonlinear trajectory across the life span. Both the PFC and NAcc are underdeveloped during childhood, as well as the functional and anatomical connections between them. The NAcc, in addition to other subcortical limbic structures, develops early in adolescence, leading to increased dopaminergic activity in this region and increased risk-taking behavior. The PFC does not fully develop until adulthood, at which point the PFC is able to more efficiently exert top-down regulatory control over bottom-up reward impulses (figure adapted from Somerville & Casey, 2010).
Adolescent reward circuitry and vulnerability to cues
Animal studies have shed light on some of the mechanisms underlying changes in reward circuitry that occur during development. For example, studies in rats have demonstrated that dopamine transmission follows an inverted U-shaped curve throughout the life span, whereby firing rates peak during adolescence (McCutcheon & Marinelli, 2009). Similarly, dopamine receptor availability increases in reward circuitry during preadolescence (Gelbard et al., 1989) and subsequently decreases during adulthood (Jucaite et al., 2010; Teicher et al., 1995). This difference in receptor availability may contribute to the increased proportion of dopaminergic neurons that activate during reward anticipation in adolescents relative to adults (Sturman & Moghaddam, 2012). Consistent with this interpretation, cross-sectional functional magnetic resonance imaging studies across development have observed heightened activity within dopaminergic regions (e.g., the ventral striatum) in response to appetitive cues (Somerville et al., 2011) among adolescents, both during reward anticipation (Geier et al., 2010) and in response to cues that predict reward outcomes (e.g., monetary reward; Galvan et al., 2006; Van Leijenhorst et al., 2010).
Importantly, increased sensitivity to environmental cues resulting from early maturation of reward circuitry allows for increased exploration and learning as adolescents progress toward adulthood and begin to establish independence. Recent work has observed enhanced reinforcement and associative learning in adolescents relative to adults (Davidow et al., 2016) as well as heightened sensitivity to feedback in dopaminergic brain regions (i.e., dorsal and ventral striatum), with both learning performance and striatal responses peaking in late adolescence in a longitudinal data set (Peters & Crone 2017). Although this boost in associative learning is generally considered to be an adaptive quality (Casey, 2015; DiMenichi & Tricomi, 2016), this improvement may also increase adolescent susceptibility to marketing. Advertisements that associate alcohol with appealing contexts and outcomes (e.g., attractive models, having fun with friends) may be more readily learned by adolescents, and further, these associations may be less easily extinguished in this population (Pattwell et al., 2012).
Beyond early maturation of reward circuitry typical across development, environmental and heritable factors are likely to contribute to variability in reward sensitivity across individuals, thereby influencing responsivity to environmental cues such as advertisements. Indeed, adolescents with alcohol use disorders exhibit heightened neural responses to advertisements for alcoholic beverages relative to nonalcoholic beverages, including in regions associated with reward processing (e.g., orbital and limbic cortices; Tapert et al., 2003). Interestingly, these responses are exacerbated in individuals with a family history of alcohol use disorders (Tapert et al., 2003), which might suggest a role of heritability in determining sensitivity to alcohol cues. Given that genetics play a key role in brain development (Giedd & Rapoport, 2010; Thompson et al., 2001) and brain function (Glahn et al., 2010; Thompson et al., 2013), it is possible that genetic variability influences the maturation and responsivity of reward circuitry. Within the food-reward domain, research has shown that children genetically at risk for obesity (via the FTO rs9939609 polymorphism) demonstrate greater activity in regions associated with reward (i.e., NAcc, OFC) in response to fast-food commercials relative to toy commercials that are rated as equally appealing (Rapuano et al., 2017) and consume more of an advertised snack (Gilbert-Diamond et al., 2017) compared with children not at risk for obesity. Although literature on developing reward circuitry has largely focused on group-level changes across the life span, further work is needed to evaluate how person-specific environmental and heritable traits influence reward sensitivity across development and whether the potential impact on reward circuits occurs before behavioral engagement (e.g., alcohol use).
Imbalance in developing reward–control circuitry and adolescent risk taking
Beyond neurodevelopmental factors that modify sensitivity to rewards, changes in the maturation of brain circuits governing inhibitory control additionally influence vulnerability to engage in risky behaviors. The PFC develops late in adolescence and contributes to the regulation of impulses and attainment of long-term goals. As such, the maturation of prefrontal control circuitry is important for establishing regulatory ability early in life. Recent longitudinal work has demonstrated a prospective relationship between adolescent brain responses during an inhibitory control task and alcohol dependence symptoms 18 months later (Mahmood et al., 2013) as well as alcohol abuse 3 years later (Wetherill et al., 2013). Further, individual differences in neural responses during inhibition failures have been shown to predict substance abuse 4 years later (Heitzeg et al., 2014). These studies indicate that dampened prefrontal control in adolescence may interfere with normative development of circuitry associated with inhibition and may contribute to increased risk taking.
The statistics on adolescent risk taking are well documented (Centers for Disease Control and Prevention, 2015). Heightened risk taking is evident not only in adolescent humans but also in rodents. Adolescent rodents exhibit increased fighting with parents, playing with peers, and drug and alcohol use (Doremus-Fitzwater et al., 2010; Logue et al., 2014), demonstrating an association between the adolescent brain and risky behavior that is independent of cultural or societal pressures. This increase in risk taking has been suggested to result from an imbalance in the developmental trajectory of the PFC (and corresponding connections) relative to the early maturation of reward circuitry (Galvan et al., 2007; Steinberg, 2007). The impact of this functional imbalance is evident in recent human work demonstrating that frontostriatal functional connectivity during an inhibitory control task predicts regulatory ability in adolescents (Somerville et al., 2011), as well as alcohol dependence severity in adults (Courtney et al., 2013). Further, a neurobehavioral model reflecting the imbalance between reward motivation and executive control has been shown to predict adolescent drug use trajectories in a longitudinal sample (Khurana et al., 2015). In addition, disrupting connectivity within this circuitry in rats affects behavioral inhibition (Meyer & Bucci, 2016) and drugseeking behaviors (Belin & Everitt, 2008), demonstrating a causal relationship between the balance between reward and control circuits and impulsivity and risk taking. This evidence suggests that a functional mismatch—combined with a heightened proclivity to engage in risky behaviors—may amplify susceptibility to alcohol advertisements and risky drinking in adolescents.
III. Social Influences on Drinking and Reward
Through repeated association with natural reinforcers such as alcohol and other drugs of abuse that directly activate brain reward circuitry, associated predictive cues (e.g., advertisements) acquire reward value and may motivate further consumption. In addition to potentially acting as a reinforcer, alcohol advertising may selectively appeal to adolescent consumers by highlighting the social benefits of drinking. In fact, alcohol advertisements are more prevalent in teen-oriented popular media than advertisements for nonalcoholic beverages and often promote youth-relevant content, such as emphasizing social and sexual cues (Austin & Hust, 2005), conveying messages of social success (Jones & Donovan, 2001), and modeling consumption by attractive and demographically similar peers (Schooler et al., 1996). By leveraging social cues that are particularly salient to adolescents, alcohol advertising may generate positive associations with alcohol from an early age. As a consequence, adolescents may develop positive expectations of alcohol even before their first drinking experience.
Social reward and risk taking in adolescence
Adolescence is marked by increasing independence and exploration, as well as enhanced social and affective engagement. As the source of social support shifts from family members to peers, adolescents take more interest in their peers and become more sensitive to social rewards and evaluations (Crone & Dahl, 2012; Foulkes & Blakemore, 2016). Social rewards—such as receiving social approval or viewing attractive faces—engage the reward system across all stages of development (Kohls et al., 2013; Ruff & Fehr, 2014), but this response is particularly strong during adolescence (Foulkes & Blakemore, 2016; Somerville et al., 2011).
In addition, nonsocial rewards such as alcohol and other drugs become more valuable for adolescents in the presence of peers (Chein et al., 2011; Foulkes & Blakemore, 2016; Thiel et al., 2008). Although adolescents show a heightened proclivity toward risky decision making in general, this is especially true when peers are present (Blakemore & Robbins, 2012; Chein et al., 2011; Steinberg, 2011). Risky behaviors during adolescence, compared with during childhood and adulthood, may be explained by social contexts amplifying reward activation (Albert et al., 2013; Chein et al., 2011; Weigard et al., 2014). For example, in a simulated driving game, adolescents who drove with a peer onlooker made riskier decisions, experienced more crashes, and exhibited greater activation in the NAcc and OFC relative to those who drove alone—a difference that did not exist for adults (Chein et al., 2011). Similarly, adolescent rodents show a preference for substances like alcohol and cocaine in the presence of peers (Logue et al., 2014; Thiel et al., 2008). Overall, adolescents appear to be especially sensitive to social contexts and peer evaluations, and these social influences may have the effect of promoting risky behavior.
Peer influences on alcohol consumption
Across species, adolescents prefer to spend time with peers (Douglas et al., 2004) and are more influenced by their behaviors and/or opinions (Knoll et al., 2015). Because adolescents are especially sensitive to peer evaluation, avoiding social rejection may be a strong motivator for initiating and continuing to drink (Somerville, 2013). Consequently, social influences on drinking can occur explicitly through peer pressure and offers to drink (Han et al., 2012), implicitly through behavior modeling (i.e., observing and imitating the behavior of other drinkers), and through established social norms (Borsari & Carey, 2001).
Explicit peer pressure, behavior modeling, and social norms all contribute to alcohol use in college students, with peer pressure and modeling directly relating to problem drinking (Wood et al., 2001). For example, in lab studies, college-age participants respond to behavior modeling by matching the heavy drinking and beverage choices of confederate drinkers, even when they are unaware of the confederate’s influence on their drinking (Borsari & Carey, 2001). Although perceived social norms and expectations may not directly contribute to problem drinking, they appear to do so indirectly through increased alcohol use among college-age students (Wood et al., 2001). Indeed, drinking habits appear to be highly susceptible to social influence and even spread through social networks, such that changes in friends’ and family members’ alcohol consumption influence an individual’s subsequent drinking (Rosenquist et al., 2010). There is substantial pressure for college students to adhere to social norms by participating in the college drinking culture. Alcohol is present at many social gatherings, and abstaining from drinking in these situations is perceived as unusual and a potential cause for exclusion from future social events (Borsari & Carey, 2001; Neighbors et al., 2006; Rabow & Duncan-Schill, 1995). Moreover, college students tend to overestimate the frequency and quantity of peers’ drinking, and in turn, their own alcohol use is influenced by this inflated perception of peer drinking (Borsari & Carey, 2001; Neighbors et al., 2006).
Because alcohol marketing implicitly and explicitly conveys a message that drinking increases social and sexual success, alcohol advertisements may particularly appeal to adolescent consumers (Jones & Donovan, 2001). By capitalizing on the hypersensitivity of the adolescent brain to social rewards, alcohol advertisements depicting salient social content may more easily grab the attention of a teenage audience and consequently accelerate reward sensitization to these cues. Even for adolescents who have not yet engaged in alcohol consumption, these advertisements may gain reward value through their conditioned association with appealing social contexts. Moreover, because drinking among adolescents and young adults often occurs in social contexts (Christiansen et al., 2002), the reward system of experienced drinkers may become sensitized to alcohol through its association with positive social contexts (e.g., lively parties). That is, alcohol advertisements that evoke positive social aspects of the drinking experience may exert pressure to begin drinking in nondrinkers or may further reinforce the behavior in experienced drinkers.
Surprisingly, little work has compared the relative influence of nonsocial alcohol advertising with advertising that highlights the social aspects of drinking (e.g., partying) on reward valuation and motivation to drink, leaving this area ripe for future research. Approximately 40% of all contemporary alcohol television commercials emphasize partying (Morgenstern et al., 2015), and these advertisements appear to be more influential for youth drinking. Across a large sample of adolescents and young adults, those with more exposure to alcohol commercials containing partying themes were more likely to have initiated drinking and binge drinking (Morgenstern et al., 2017). Given that the tobacco industry was admonished for using similar advertising campaigns that specifically appealed to youth populations (e.g., Joe Camel), these areas of research raise important policy considerations regarding alcohol advertising to youth (e.g., limiting depictions of social drinking to youth audiences; Cohen, 2000; DiFranza et al., 1991).
IV. Reward Sensitization to Alcohol Cues
Although the adolescent proclivity toward risk taking and sensitivity to social pressures may encourage alcohol experimentation, favorable experiences with drinking may amplify the salience of alcohol-predictive cues (e.g., alcohol advertisements) and increase the likelihood of drinking upon future exposure to these cues. This association is established through a process of reward learning whereby pleasurable experiences endow alcohol cues with motivational salience for drinkers. In other words, the experience of intoxication that accompanies drinking reinforces continued drinking as reward circuitry becomes sensitized to cues that predict this experience, which may ultimately contribute to patterns of risky alcohol use (e.g., binge drinking episodes).
Drinking sensitizes reward circuitry to alcohol cues
Alcohol consumption activates the brain’s reward circuitry, triggering a process of reward learning and reinforcement that motivates continued consumption (Robinson & Berridge, 1993; Wise, 1998). Specifically, the ethanol in an alcoholic beverage triggers mesolimbic dopamine release in reward circuitry—extending from the ventral tegmental area (VTA) to the NAcc and beyond (Brodie et al., 1999; Spanagel & Weiss, 1999). This release of dopamine initiates a process of reward learning that (a) strengthens the association between the reward-predictive cue (e.g., alcohol advertisements or a brand logo) and the reward outcome and (b) sets the motivational value of the cue, thereby increasing how much it is “wanted” on subsequent encounters (Berridge & Robinson, 2003; Volkow & Morales, 2015). Over time, the response patterns in dopamine neurons shift such that firing rates are time-locked to the presence of reliable reward-predictive cues, thereby reflecting the expectation of reward rather than the receipt of the reward itself (Berridge & Robinson, 1998; Schultz, 1998; Schultz et al., 1997).
Furthermore, the strength of the dopamine response scales with the incentive salience of the cue and motivates consumption of the associated reward (Berridge & Robinson, 1998; Robinson & Berridge, 1993). Because of this relationship, human neuroimaging studies have successfully used cue-reactivity paradigms to relate responses to images of alcohol, other drugs, or food within reward circuitry to the strength of subjectively experienced cravings (Kühn & Gallinat, 2011) and to real-world consumption and related health outcomes (Courtney et al., 2018c; Demos et al., 2012; Rapuano et al., 2016). This behaviorally relevant reward response occurs in individuals who have, over time, learned a reliable and salient association between predictive cues (e.g., alcohol advertisements) and corresponding rewards (e.g., the drinking experience).
Importantly, this dopamine-dependent motivational system is distinct from the system underlying hedonic pleasure (Berridge & Robinson, 1998, 2003). As a result, once a strong association is established, salient alcohol cues such as appealing advertisements may compel individuals to drink whether or not they report enjoying alcohol. This theory of incentive-sensitization in addiction (Robinson & Berridge, 2001, 2008) may explain how reward associations that developed during initial experimentation with alcohol can eventually lead to alcohol abuse through continued heavy drinking.
Reward system plasticity and alcohol dependence
Because adolescence is a sensitive window of brain development, alcohol consumption during this developmental period may interfere with normative neuromaturation (Bava & Tapert, 2010; Squeglia et al., 2009) by compromising white matter connectivity (McQueeny et al., 2009) and prefrontal cortical volume (De Bellis et al., 2005; Medina et al., 2008). These maturational disruptions may result in a cascade of neurocognitive impairments (Bava & Tapert, 2010; Squeglia et al., 2009) and increase the likelihood of later alcohol and substance dependence (Squeglia et al., 2009; Zeigler et al., 2005). More specifically, disruptions in prefrontal cortical development resulting from heavy alcohol use during adolescence may contribute to deficits in behavioral inhibition and exaggerated risk taking by weakening prefrontal contributions to behavior (Crews et al., 2007). In an experimentally induced parallel, a chemogenetic reversal in the balance of PFC control over the NAcc impaired behavioral inhibition in adolescent rats (Meyer & Bucci, 2016), pointing to a sensitive window for the development of prefrontal-dependent inhibitory control that may be disrupted by early alcohol use.
In response to long-term drinking, reward circuitry undergoes lasting neuroplastic changes that increase sensitivity to alcohol-predictive cues and decrease sensitivity to nondrug rewards. In contrast to natural rewards, drug rewards such as ethanol elicit greater and more sustained dopamine release and acquire excessive motivational salience (Kalivas & O’Brien, 2008). Moreover, morphological changes resulting from frequent alcohol use (e.g., increased receptor density in reward circuitry; Koob & Volkow, 2010; Stuber et al., 2010; Volkow & Morales, 2015) may further contribute to increased sensitivity to alcohol cues and explain how social drinking may progress toward dependent drinking for some individuals (Robinson & Berridge, 1993, 2001). Reward responses to alcohol cues in dependent drinkers have been shown to be exaggerated (Tapert et al., 2003); persistent (i.e., marked by decreased habituation to cue repetition; Dager et al., 2013); and associated with biased attention toward alcohol cues (Robinson & Berridge, 2008), increased alcohol craving (Kühn & Gallinat, 2011), and compulsive drinking (Koob & Volkow, 2010; Robinson & Berridge, 1993).
Differences in prefrontal–reward circuitry resulting from frequent alcohol abuse may contribute to an increased risk of dependence later in life. According to the Impaired Response Inhibition and Salience Attribution (I-RISA) model of addiction, shifts in the balance of prefrontal–reward circuitry reinforce the cycle of intoxication, craving, bingeing, and withdrawal that occurs during dependence (Goldstein & Volkow, 2002). The excessive motivational salience assigned to drug cues during intoxication leads to an overvaluation of drug rewards, which manifests as craving, and contributes to inhibitory control failure and compulsive drug pursuit. Drug and alcohol cues are resistant to devaluation; therefore, over time, drug and alcohol pursuit can become habitual rather than goal directed (Dickinson et al., 2002). That is, exposure to associated cues may compel drug use or drinking even when the drugs are associated with aversive outcomes or come at the expense of adaptive behaviors (Robinson & Berridge, 2001). The excessive approach tendency that addicts display toward reward-predictive cues mirrors the maladaptive sign-tracking behavior exhibited by some conditioned animals, a pattern in which the animal favors and perseverates on a conditioned cue instead of the associated reward (Robinson & Berridge, 2008; Tomie et al., 2008; Tomie & Sharma, 2014). Sign-tracking rats may exhibit excessive approach, contact, and consumption-related behaviors (e.g., gnawing) toward a food-predictive lever, even in the presence of the food pellet itself—demonstrating the compulsive and sometimes maladaptive nature of reward learning. Similarly, heavy drinkers are quicker to approach alcohol-related cues (e.g., a picture of someone drinking alcohol) than light drinkers (Field et al., 2008). Both sign-tracking and drug dependence may rely on an overexpression of dopamine in reward circuitry and an excessive attribution of motivational salience to the cue that compels its consumption (Tomie et al., 2008).
V. Discussion of Neurobiological Plausibility
Epidemiological data have consistently demonstrated evidence for an association between alcohol advertisement exposure and drinking behavior (Anderson et al., 2009; Hastings et al., 2005; McClure et al., 2013; Smith & Foxcroft, 2009; Snyder et al., 2006). However, policy recommendations and early prevention strategies may benefit from a narrower understanding of the specific factors that contribute to this relationship. Although research has called into question the utility of neural responses to alcohol advertisements for the purpose of understanding drinking outcomes (Courtney et al., 2018b; Meyer, 2018), we argue that neurobiological associations, in conjunction with strong epidemiological evidence, may help to elucidate the “black box” underlying alcohol advertisement exposure and observable outcomes (Fedak et al., 2015). To understand how advertisements may lead to risky drinking behavior, we have contextualized this association in the literature on neurodevelopment and addiction and have proposed a conceptual model that integrates correlational data with plausible neurobiological mechanisms that may underlie this association (Figure 1).
More specifically, the proposed model relates reward sensitization to alcohol advertising, adolescent brain development, and cue-motivated consumption in order to highlight biologically informed routes by which marketing strategies may exploit neural processes and vulnerabilities. Given that a limited number of studies have directly approached the relationship between exposure to alcohol advertisements and risky drinking, this review has drawn inferences from the broader literature to propose a unified framework. Although this model provides an initial starting point in considering a neurobiological mechanism underlying the association between alcohol advertisement exposure and underage drinking, it also brings remaining questions into focus. We propose directions for future research (Box 1) that would extend previous work and test specific hypotheses targeting characteristics of alcohol advertisements that appeal to adolescents and additional factors promoting sensitivity to alcohol cues. Because age-related interests and neurobiological vulnerabilities may interact with specific features of advertisements to predict sensitivity to drinking, the influence of alcohol marketing strategies on the developing brain should be disentangled and used to inform public policy.
BOX 1.
Directions for future research

Our model suggests that alcohol advertising may inordinately appeal to adolescent consumers by emphasizing salient themes during a window of brain development characterized by sensitivity to socioenvironmental cues. Accordingly, enacting policies that shield youth from adolescent-relevant alcohol advertising until they emerge from this sensitive window of development (around age 21 years) might be a particularly effective strategy for preventing early alcohol abuse. For example, this policy recommendation could be implemented by imposing regulations on the frequency or presence of alcohol advertisements on television shows and social media pages that are predominantly visited by individuals younger than age 21. Another regulation on alcohol marketing might consider requiring models in advertisements to appear older than a certain age in order to strategically limit adolescent exposure to advertisements that portray young adults in risky contexts that appeal to adolescents (e.g., loud parties). In addition, educational interventions such as counter-advertising or programs that appeal to adolescent values, such as autonomy, by casting them as targets of advertising campaigns might motivate voluntary resistance to marketing ploys (Bryan et al., 2016) and reduce rates of adolescent alcohol abuse. Similarly, intervention programs that embrace adolescent sensitivity to social norms and peer evaluations might attempt to shift perceptions of drinking norms by highlighting messaging from peer ambassadors to drink responsibly. By identifying specific ways in which alcohol marketing shifts the functional balance of prefrontal–reward circuitry to promote risky drinking behaviors in youth, future research can inform policies directed toward the most effective prevention and intervention strategies.
Acknowledgements
The authors thank Jim Sargent for valuable discussions and feedback on the scope of the review.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grant R01AA021347, National Institute on Drug Abuse Grants R01DA022582 and U01DA041174, National Institutes of Health National Research Service Award F31MH111192, and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK97399. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Acikalin M. Y., Watson K. K., Fitzsimons G. J., Platt M. L. Rhesus macaques form preferences for brand logos through sex and social status based advertising. PLoS One. 2018;13(2):e0193055. doi: 10.1371/journal.pone.0193055. doi:10.1371/journal.pone.0193055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert D., Chein J., Steinberg L. The teenage brain: Peer influences on adolescent decision making. Current Directions in Psychological Science. 2013;22:114–120. doi: 10.1177/0963721412471347. doi:10.1177/0963721412471347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., de Bruijn A., Angus K., Gordon R., Hastings G. Impact of alcohol advertising and media exposure on adolescent alcohol use: a systematic review of longitudinal studies. Alcohol and Alcoholism. 2009;44:229–243. doi: 10.1093/alcalc/agn115. doi:10.1093/alcalc/agn115. [DOI] [PubMed] [Google Scholar]
- Austin E. W., Hust S. J. T. Targeting adolescents? The content and frequency of alcoholic and nonalcoholic beverage ads in magazine and video formats November 1999-April 2000. Journal of Health Communication. 2005;10:769–785. doi: 10.1080/10810730500326757. doi:10.1080/10810730500326757. [DOI] [PubMed] [Google Scholar]
- Bartholow B. D., Loersch C., Ito T. A., Levsen M. P., Volpert-Esmond H. I., Fleming K. A., Carter B. K. University-affiliated alcohol marketing enhances the incentive salience of alcohol cues. Psychological Science. 2018;29:83–94. doi: 10.1177/0956797617731367. doi:10.1177/0956797617731367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Tapert S. F. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsychology Review. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. doi:10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer Institute. Advertising and marketing code. 2015. http://www.beerinstitute.org/wp-content/uploads/2016/11/2015-Beer-Ad-Code-Brochure.pdf Retrieved from. [Google Scholar]
- Belin D., Everitt B. J. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. doi:10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Berkman E. T., Falk E. B. Beyond brain mapping: Using neural measures to predict real-world outcomes. Current Directions in Psychological Science. 2013;22:45–50. doi: 10.1177/0963721412469394. doi:10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K. C., Robinson T. E. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. doi:10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge K. C., Robinson T. E. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. doi:10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Choudhury S. Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. doi:10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Robbins T. W. Decision-making in the adolescent brain. Nature Neuroscience. 2012;15:1184–1191. doi: 10.1038/nn.3177. doi:10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Borsari B., Carey K. B. Peer influences on college drinking: A review of the research. Journal of Substance Abuse. 2001;13:391–424. doi: 10.1016/s0899-3289(01)00098-0. doi:10.1016/S0899-3289(01)00098-0. [DOI] [PubMed] [Google Scholar]
- Brenhouse H. C., Andersen S. L. Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neuroscience and Biobehavioral Reviews. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. doi:10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie M. S., Pesold C., Appel S. B. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcoholism: Clinical and Experimental Research. 1999;23:1848–1852. doi:10.1111/j.1530-0277.1999.tb04082.x. [PubMed] [Google Scholar]
- Bruce A. S., Bruce J. M., Black W. R., Lepping R. J., Henry J. M., Cherry J. B. C., Savage C. R. Branding and a child’s brain: An fMRI study of neural responses to logos. Social Cognitive and Affective Neuroscience. 2014;9:118–122. doi: 10.1093/scan/nss109. doi:10.1093/scan/nss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. S., Holsen L. M., Chambers R. J., Martin L. E., Brooks W. M., Zarcone J. R., Savage C. R. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity. 2010;34:1494–1500. doi: 10.1038/ijo.2010.84. doi:10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A. S., Lepping R. J., Bruce J. M., Cherry J. B. C., Martin L. E., Davis A. M., Savage C. R. Brain responses to food logos in obese and healthy weight children. Journal of Pediatrics. 2013;162:759–764. e2. doi: 10.1016/j.jpeds.2012.10.003. doi:10.1016/j.jpeds.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Bryan C. J., Yeager D. S., Hinojosa C. P., Chabot A., Bergen H., Kawamura M., Steubing F. Harnessing adolescent values to motivate healthier eating. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:10830–10835. doi: 10.1073/pnas.1604586113. doi:10.1073/pnas.1604586113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B. J. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. doi:10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey B. J., Heller A. S., Gee D. G., Cohen A. O. Development of the emotional brain. Neuroscience Letters. 2019;693:29–34. doi: 10.1016/j.neulet.2017.11.055. doi:10.1016/j.neulet.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Youth Risk Behavior Surveillance System (YRBSS) 2015. Retrieved from www.cdc.gov/yrbs. [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. doi:10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen M., Vik P. W., Jarchow A. College student heavy drinking in social contexts versus alone. Addictive Behaviors. 2002;27:393–404. doi: 10.1016/s0306-4603(01)00180-0. doi:10.1016/S0306-4603(01)00180-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. B. Playing to win: Marketing and public policy at odds over Joe Camel. Journal of Public Policy & Marketing. 2000;19:155–167. doi:10.1509/jppm.19.2.155.17123. [Google Scholar]
- Courtney A. L., PeConga E. K., Wagner D. D., Rapuano K. M. Calorie information and dieting status modulate reward and control activation during the evaluation of food images. PLoS One. 2018a;13(11):e0204744. doi: 10.1371/journal.pone.0204744. doi:10.1371/journal.pone.0204744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney A. L., Rapuano K. M., Sargent J. D., Heatherton T. F., Kelley W. M. Brain reward responses are behaviorally relevant: The authors respond. Journal of Studies on Alcohol and Drugs. 2018b;79:41–42. doi: 10.15288/jsad.2018.79.41. doi:10.15288/jsad.2018.79.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney A. L., Rapuano K. M., Sargent J. D., Heatherton T. F., Kelley W. M. Reward system activation in response to alcohol advertisements predicts college drinking. Journal of Studies on Alcohol and Drugs. 2018c;79:29–38. doi: 10.15288/jsad.2018.79.29. doi:10.15288/jsad.2018.79.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K. E., Ghahremani D. G., Ray L. A. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addiction Biology. 2013;18:593–604. doi: 10.1111/adb.12013. doi:10.1111/adb.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F., He J., Hodge C. Adolescent cortical development: A critical period of vulnerability for addiction. Pharmacology, Biochemistry, and Behavior. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. doi:10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E. A., Dahl R. E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. doi:10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dager A. D., Anderson B. M., Stevens M. C., Pulido C., Rosen R., Jiantonio-Kelly R. E., Pearlson G. D. Influence of alcohol use and family history of alcoholism on neural response to alcohol cues in college drinkers. Alcoholism: Clinical and Experimental Research. 2013;37(Supplement 1):E161–E171. doi: 10.1111/j.1530-0277.2012.01879.x. doi:10.1111/j.1530-0277.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S. P., Munafò M. R., Johansen-Berg H., Smith S. M., Rogers R. D., Matthews P. M., Walton R. T. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. doi:10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidow J. Y., Foerde K., Galván A., Shohamy D. An upside to reward sensitivity: The hippocampus supports enhanced reinforcement learning in adolescence. Neuron. 2016;92:93–99. doi: 10.1016/j.neuron.2016.08.031. doi:10.1016/j.neuron.2016.08.031. [DOI] [PubMed] [Google Scholar]
- De Bellis M. D., Narasimhan A., Thatcher D. L., Keshavan M. S., Soloff P., Clark D. B. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. doi:10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Demos K. E., Heatherton T. F., Kelley W. M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. Journal of Neuroscience. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. doi:10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A., Wood N., Smith J. W. Alcohol seeking by rats: Action or habit? Quarterly Journal of Experimental Psychology. B, Comparative and Physiological Psychology. 2002;55:331–348. doi: 10.1080/0272499024400016. doi:10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- DiFranza J. R., Richards J. W., Paulman P. M., Wolf-Gillespie N., Fletcher C., Jaffe R. D., Murray D. RJR Nabisco’s cartoon camel promotes camel cigarettes to children. JAMA. 1991;266:3149–3153. doi:10.1001/jama.1991.03470220065028. [PubMed] [Google Scholar]
- DiMenichi B. C., Tricomi E. Are you smarter than a teenager? Maybe not when it comes to reinforcement learning. Neuron. 2016;92:1–3. doi: 10.1016/j.neuron.2016.09.043. doi:10.1016/j.neuron.2016.09.043. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater T. L., Varlinskaya E. I., Spear L. P. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. doi:10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L. A., Varlinskaya E. I., Spear L. P. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Developmental Psychobiology. 2004;45:153–162. doi: 10.1002/dev.20025. doi:10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Fedak K. M., Bernal A., Capshaw Z. A., Gross S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerging Themes in Epidemiology. 2015;12:14. doi: 10.1186/s12982-015-0037-4. doi:10.1186/s12982-015-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Kiernan A., Eastwood B., Child R. Rapid approach responses to alcohol cues in heavy drinkers. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39:209–218. doi: 10.1016/j.jbtep.2007.06.001. doi:10.1016/j.jbtep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Foulkes L., Blakemore S.-J. Is there heightened sensitivity to social reward in adolescence? Current Opinion in Neurobiology. 2016;40:81–85. doi: 10.1016/j.conb.2016.06.016. doi:10.1016/j.conb.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T. A., Parra C. E., Penn J., Voss H., Glover G., Casey B. J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. doi:10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T., Voss H., Glover G., Casey B. J. Risk-taking and the adolescent brain: Who is at risk? Developmental Science. 2007;10:F8–F14. doi: 10.1111/j.1467-7687.2006.00579.x. doi:10.1111/j.1467-7687.2006.00579.x. [DOI] [PubMed] [Google Scholar]
- Gearhardt A. N., Yokum S., Stice E., Harris J. L., Brownell K. D. Relation of obesity to neural activation in response to food commercials. Social Cognitive and Affective Neuroscience. 2014;9:932–938. doi: 10.1093/scan/nst059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C. F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. doi:10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbard H. A., Teicher M. H., Faedda G., Baldessarini R. J. Postnatal development of dopamine D1 and D2 receptor sites in rat striatum. Developmental Brain Research. 1989;49:123–130. doi: 10.1016/0165-3806(89)90065-5. doi:10.1016/0165-3806(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Giedd J. N., Rapoport J. L. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. doi:10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D., Emond J. A., Lansigan R. K., Rapuano K. M., Kelley W. M., Heatherton T. F., Sargent J. D. Television food advertisement exposure and FTO rs9939609 genotype in relation to excess consumption in children. International Journal of Obesity. 2017;41:23. doi: 10.1038/ijo.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn D. C., Winkler A. M., Kochunov P., Almasy L., Duggirala R., Carless M. A., Blangero J. Genetic control over the resting brain. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. Z., Volkow N. D. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. doi:10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. F., Dawson D. A. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. doi:10.1016/S0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Green B. N., Johnson C. D., Adams A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. Journal of Chiropractic Medicine. 2006;5:101–117. doi: 10.1016/S0899-3467(07)60142-6. doi:10.1016/S0899-3467(07)60142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube J. W., Wallack L. Television beer advertising and drinking knowledge, beliefs, and intentions among schoolchildren. American Journal of Public Health. 1994;84:254–259. doi: 10.2105/ajph.84.2.254. doi:10.2105/AJPH.84.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Grogan-Kaylor A., Delva J., Castillo M. The role of peers and parents in predicting alcohol consumption among Chilean youth. International Journal of Child and Adolescent Health. 2012;5:53–64. [PMC free article] [PubMed] [Google Scholar]
- Hastings G., Anderson S., Cooke E., Gordon R. Alcohol marketing and young people’s drinking: A review of the research. Journal of Public Health Policy. 2005;26:296–311. doi: 10.1057/palgrave.jphp.3200039. doi:10.1057/palgrave.jphp.3200039. [DOI] [PubMed] [Google Scholar]
- Hawkins J. D., Graham J. W., Maguin E., Abbott R., Hill K. G., Catalano R. F. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of Studies on Alcohol. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. doi:10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg M. M., Nigg J. T., Hardee J. E., Soules M., Steinberg D., Zubieta J.-K., Zucker R. A. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug and Alcohol Dependence. 2014;141:51–57. doi: 10.1016/j.drugalcdep.2014.05.002. doi:10.1016/j.drugalcdep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. C., Donovan R. J. Messages in alcohol advertising targeted to youth. Australian and New Zealand Journal of Public Health. 2001;25:126–131. doi: 10.1111/j.1753-6405.2001.tb01833.x. doi:10.1111/j.1753-6405.2001.tb01833.x. [DOI] [PubMed] [Google Scholar]
- Jucaite A., Forssberg H., Karlsson P., Halldin C., Farde L. Age-related reduction in dopamine D1 receptors in the human brain: From late childhood to adulthood, a positron emission tomography study. Neuroscience. 2010;167:104–110. doi: 10.1016/j.neuroscience.2010.01.034. doi:10.1016/j.neuroscience.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W., O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. doi:10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Khurana A., Romer D., Betancourt L. M., Brodsky N. L., Giannetta J. M., Hurt H. Experimentation versus progression in adolescent drug use: A test of an emerging neurobehavioral imbalance model. Development and Psychopathology. 2015;27:901–913. doi: 10.1017/S0954579414000765. doi:10.1017/S0954579414000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L. J., Magis-Weinberg L., Speekenbrink M., Blakemore S.-J. Social influence on risk perception during adolescence. Psychological Science. 2015;26:583–592. doi: 10.1177/0956797615569578. doi:10.1177/0956797615569578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G., Perino M. T., Taylor J. M., Madva E. N., Cayless S. J., Troiani V., Schultz R. T. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia. 2013;51:2062–2069. doi: 10.1016/j.neuropsychologia.2013.07.020. doi:10.1016/j.neuropsychologia.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G. F., Volkow N. D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. doi:10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. European Journal of Neuroscience. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. doi:10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Logue S., Chein J., Gould T., Holliday E., Steinberg L. Adolescent mice, unlike adults, consume more alcohol in the presence of peers than alone. Developmental Science. 2014;17:79–85. doi: 10.1111/desc.12101. doi:10.1111/desc.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez R. B., Hofmann W., Wagner D. D., Kelley W. M., Heatherton T. F. Neural predictors of giving in to temptation in daily life. Psychological Science. 2014;25:1337–1344. doi: 10.1177/0956797614531492. doi:10.1177/0956797614531492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood O. M., Goldenberg D., Thayer R., Migliorini R., Simmons A. N., Tapert S. F. Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addictive Behaviors. 2013;38:1435–1441. doi: 10.1016/j.addbeh.2012.07.012. doi:10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure A. C., Tanski S. E., Gilbert-Diamond D., Adachi-Mejia A. M., Li Z., Li Z., Sargent J. D. Receptivity to television fast-food restaurant marketing and obesity among U.S. youth. American Journal of Preventive Medicine. 2013;45:560–568. doi: 10.1016/j.amepre.2013.06.011. doi:10.1016/j.amepre.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon J. E., Marinelli M. Age matters. European Journal of Neuroscience. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. doi:10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T., Schweinsburg B. C., Schweinsburg A. D., Jacobus J., Bava S., Frank L. R., Tapert S. F. Altered white matter integrity in adolescent binge drinkers. Alcoholism: Clinical and Experimental Research. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. doi:10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina K. L., McQueeny T., Nagel B. J., Hanson K. L., Schweinsburg A. D., Tapert S. F. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcoholism: Clinical and Experimental Research. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. doi:10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H. C., Bucci D. J. Imbalanced activity in the orbitofrontal cortex and nucleus accumbens impairs behavioral inhibition. Current Biology. 2016;26:2834–2839. doi: 10.1016/j.cub.2016.08.034. doi:10.1016/j.cub.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. E. Back to the future . . . or . . . is that all there is? A commentary on Courtney et al. (2018) Journal of Studies on Alcohol and Drugs. 2018;79:39–40. doi:10.15288/jsad.2018.79.39. [PubMed] [Google Scholar]
- Mills K. L., Goddings A.-L., Clasen L. S., Giedd J. N., Blakemore S.-J. The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience. 2014;36:147–160. doi: 10.1159/000362328. doi:10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Morgenstern M., Schoeppe F., Campbell J., Braam M. W. G., Stoolmiller M., Sargent J. D. Content themes of alcohol advertising in U.S. television-latent class analysis. Alcoholism: Clinical and Experimental Research. 2015;39:1766–1774. doi: 10.1111/acer.12811. doi:10.1111/acer.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern M., Li Z., Li Z., Sargent J. D. The party effect: Prediction of future alcohol use based on exposure to specific alcohol advertising content. Addiction. 2017;112:63–70. doi: 10.1111/add.13509. doi:10.1111/add.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbors C., Dillard A. J., Lewis M. A., Bergstrom R. L., Neil T. A. Normative misperceptions and temporal precedence of perceived norms and drinking. Journal of Studies on Alcohol. 2006;67:290–299. doi: 10.15288/jsa.2006.67.290. doi:10.15288/jsa.2006.67.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J., Stice E., Yokum S., Bohon C. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite. 2011;57:65–72. doi: 10.1016/j.appet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noël X., Van Der Linden M., Bechara A. The neurocognitive mechanisms of decision-making, impulse control, and loss of willpower to resist drugs. Psychiatry. 2006;3:30–41. [PMC free article] [PubMed] [Google Scholar]
- Pattwell S. S., Duhoux S., Hartley C. A., Johnson D. C., Jing D., Elliott M. D., Lee F. S. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S., Crone E. A. Increased striatal activity in adolescence benefits learning. Nature Communications. 2017;8:1983. doi: 10.1038/s41467-017-02174-z. doi:10.1038/s41467-017-02174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H., O’Doherty J., Shiv B., Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proceedings of the National Academy of Sciences. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. A., Kendler K. S. Age at first drink and risk for alcoholism: A noncausal association. Alcoholism: Clinical and Experimental Research. 1999;23:101–107. doi:10.1097/00000374-199901000-00014. [PubMed] [Google Scholar]
- Rabow J., Duncan-Schill M. Drinking among college students. Journal of Alcohol and Drug Education. 1995;40:52–64. [Google Scholar]
- Rapuano K. M., Huckins J. F., Sargent J. D., Heatherton T. F., Kelley W. M. Individual differences in reward and somatosensory-motor brain regions correlate with adiposity in adolescents. Cerebral Cortex. 2016;26:2602–2611. doi: 10.1093/cercor/bhv097. doi:10.1093/cercor/bhv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapuano K. M., Zieselman A. L., Kelley W. M., Sargent J. D., Heatherton T. F., Gilbert-Diamond D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:160–165. doi: 10.1073/pnas.1605548113. doi:10.1073/pnas.1605548113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. doi:10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. doi:10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Berridge K. C. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. doi:10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist J. N., Murabito J., Fowler J. H., Christakis N. A. The spread of alcohol consumption behavior in a large social network. Annals of Internal Medicine. 2010;152:426–433. doi: 10.1059/0003-4819-152-7-201004060-00007. W141. doi:10.7326/0003-4819-152-7-201004060-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff C. C., Fehr E. The neurobiology of rewards and values in social decision making. Nature Reviews Neuroscience. 2014;15:549–562. doi: 10.1038/nrn3776. doi:10.1038/nrn3776. [DOI] [PubMed] [Google Scholar]
- Schacht J. P., Anton R. F., Myrick H. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addiction Biology. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. doi:10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler C., Basil M. D., Altman D. G. Alcohol and cigarette advertising on billboards: Targeting with social cues. Health Communication. 1996;8:109–129. doi:10.1207/s15327027hc0802_1. [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. doi:10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W., Dayan P., Montague P. R. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. doi:10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Smith L. A., Foxcroft D. R. The effect of alcohol advertising, marketing and portrayal on drinking behaviour in young people: Systematic review of prospective cohort studies. BMC Public Health. 2009;9:51. doi: 10.1186/1471-2458-9-51. doi:10.1186/1471-2458-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder L. B., Milici F. F., Slater M., Sun H., Strizhakova Y. Effects of alcohol advertising exposure on drinking among youth. Archives of Pediatrics & Adolescent Medicine. 2006;160:18–24. doi: 10.1001/archpedi.160.1.18. doi:10.1001/archpedi.160.1.18. [DOI] [PubMed] [Google Scholar]
- Somerville L. H. Special issue on the teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science. 2013;22:121–127. doi: 10.1177/0963721413476512. doi:10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L. H., Casey B. J. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. doi:10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L. H., Hare T., Casey B. J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. doi:10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R., Weiss F. The dopamine hypothesis of reward: Past and current status. Trends in Neurosciences. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. doi:10.1016/S0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Squeglia L. M., Jacobus J., Tapert S. F. The influence of substance use on adolescent brain development. Clinical EEG and Neuroscience. 2009;40:31–38. doi: 10.1177/155005940904000110. doi:10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: New perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16:55–59. doi:10.1111/j.1467-8721.2007.00475.x. [Google Scholar]
- Steinberg L. Adolescent risk taking: A social neuroscience perspective. In: Amsel E., Smetana J., editors. Adolescent vulnerabilities and opportunities. Cambridge, England: Cambridge University Press; 2011. pp. 41–64. doi:10.1017/CBO9781139042819.005. [Google Scholar]
- Stoeckel L. E., Weller R. E., Cook E. W., III, Twieg D. B., Knowlton R. C., Cox J. E. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–647. doi: 10.1016/j.neuroimage.2008.02.031. doi:10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stuber G. D., Woodward Hopf F., Tye K. M., Chen B. T., Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Current Topics in Behavioral Neurosciences. 2010;3:3–27. doi: 10.1007/7854_2009_23. doi:10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- Sturman D. A., Moghaddam B. Striatum processes reward differently in adolescents versus adults. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:1719–1724. doi: 10.1073/pnas.1114137109. doi:10.1073/pnas.1114137109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D. W., Fellows L. K., Small D. M., Dagher A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiology & Behavior. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. doi:10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tanski S. E., McClure A. C., Jernigan D. H., Sargent J. D. Alcohol brand preference and binge drinking among adolescents. Archives of Pediatrics & Adolescent Medicine. 2011;165:675–676. doi: 10.1001/archpediatrics.2011.113. doi:10.1001/archpediatrics.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert S. F., Cheung E. H., Brown G. G., Frank L. R., Paulus M. P., Schweinsburg A. D., Brown S. A. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. doi:10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Teicher M. H., Andersen S. L., Hostetter J. C., Jr. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. doi:10.1016/0165-3806(95)00109-Q. [DOI] [PubMed] [Google Scholar]
- Thiel K. J., Okun A. C., Neisewander J. L. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug and Alcohol Dependence. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. doi:10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P. M., Cannon T. D., Narr K. L., van Erp T., Poutanen V.-P., Huttunen M., Toga A. W. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. doi:10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson P. M., Ge T., Glahn D. C., Jahanshad N., Nichols T. E. Genetics of the connectome. NeuroImage. 2013;80:475–488. doi: 10.1016/j.neuroimage.2013.05.013. doi:10.1016/j.neuroimage.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A., Grimes K. L., Pohorecky L. A. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. doi:10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomie A., Sharma N. Pavlovian sign-tracking model of alcohol abuse. Current Drug Abuse Reviews. 2013;6:201–219. doi: 10.2174/18744737113069990023. doi:10.2174/18744737113069990023. [DOI] [PubMed] [Google Scholar]
- van der Laan L. N., de Ridder D. T. D., Viergever M. A., Smeets P. A. M. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. NeuroImage. 2011;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. doi:10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L., Gunther Moor B., Op de Macks Z. A., Rombouts S. A. R. B., Westenberg P. M., Crone E. A. Adolescent risky decision-making: Neurocognitive development of reward and control regions. NeuroImage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. doi:10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Volkow N. D., Morales M. The brain on drugs: From reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. doi:10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Dal Cin S., Sargent J. D., Kelley W. M., Heatherton T. F. Spontaneous action representation in smokers when watching movie characters smoke. Journal of Neuroscience. 2011;31:894–898. doi: 10.1523/JNEUROSCI.5174-10.2011. doi:10.1523/JNEUROSCI.5174-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigard A., Chein J., Albert D., Smith A., Steinberg L. Effects of anonymous peer observation on adolescents’ preference for immediate rewards. Developmental Science. 2014;17:71–78. doi: 10.1111/desc.12099. doi:10.1111/desc.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill R. R., Squeglia L. M., Yang T. T., Tapert S. F. A longitudinal examination of adolescent response inhibition: Neural differences before and after the initiation of heavy drinking. Psychopharmacology. 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. doi:10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. J., Sayette M. A., Fiez J. A. Prefrontal responses to drug cues: A neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. doi:10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. A. Drug-activation of brain reward pathways. Drug and Alcohol Dependence. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. doi:10.1016/S0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Wise R. A., Rompre P. P. Brain dopamine and reward. Annual Review of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. doi:10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wood M. D., Read J. P., Palfai T. P., Stevenson J. F. Social influence processes and college student drinking: The mediational role of alcohol outcome expectancies. Journal of Studies on Alcohol. 2001;62:32–43. doi: 10.15288/jsa.2001.62.32. doi:10.15288/jsa.2001.62.32. [DOI] [PubMed] [Google Scholar]
- Zeigler D. W., Wang C. C., Yoast R. A., Dickinson B. D., McCaffree M. A., Robinowitz C. B., Sterling M. L. & the Council on Scientific Affairs, American Medical Association. The neurocognitive effects of alcohol on adolescents and college students. Preventive Medicine. 2005;40:23–32. doi: 10.1016/j.ypmed.2004.04.044. doi:10.1016/j.ypmed.2004.04.044. [DOI] [PubMed] [Google Scholar]