Abstract
Aging is associated with increased oxidative stress in vascular endothelial and smooth muscle cells, which contribute to the development of a wide range of diseases affecting the circulatory system in older adults. There is growing evidence that in addition to increased production of reactive oxygen species (ROS), aging critically impairs pathways determining cellular resilience to oxidative stressors. In young organisms, the evolutionarily conserved nuclear factor-erythroid-2-related factor 2 (Nrf2)-mediated antioxidant response pathway maintains cellular reduction-oxidation homeostasis and promotes a youthful cellular phenotype by regulating the transcription of an array of cytoprotective (antioxidant, pro-survival, anti-inflammatory and macromolecular damage repair) genes. A critical mechanism by which increased ROS production and Nrf2 dysfunction promote vascular aging and exacerbate pathogenesis of age-related vascular diseases is induction of cellular senescence, an evolutionarily conserved cellular stress response mechanism. Senescent cells cease dividing and undergo distinctive phenotypic alterations, contributing to impairment of angiogenic processes, chronic sterile inflammation, remodeling of the extracellular matrix, and barrier dysfunction. Herein, we review mechanisms contributing to dysregulation of Nrf2-driven cytoprotective responses in the aged vasculature and discuss the multifaceted role of Nrf2 dysfunction in the genesis of age-related pathologies affecting the circulatory system, including its role in induction of cellular senescence. Therapeutic strategies that restore Nrf2 signaling and improve vascular resilience in aging are explored to reduce cardiovascular mortality and morbidity in older adults.
Keywords: Senescence, Reactive oxygen species, Oxidative stress, Antioxidant, Stress resistance, Vascular cognitive impairment, Vascular aging, Atherosclerosis, Nrf2 deficiency, Nrf2 dysfunction
Introduction
Epidemiological, clinical, and experimental studies demonstrate that advanced age per se promotes the pathogenesis of a wide range of diseases affecting the circulatory system (Ungvari et al. 2018a, b). Pathophysiological consequences of intrinsic vascular aging are the leading cause of morbidity and mortality among patients over 65 years of age and are major cause for the age-related decline in physical health-related quality of life. It is well established in the literature that aging-induced oxidative macromolecular damage and oxidative stress-mediated proinflammatory signaling pathways in cells within the vascular wall are important determinants of increased disease susceptibility in older adults, contributing to the pathogenesis of hypertension, stroke, coronary heart disease, heart failure, dysregulation of cerebral and peripheral blood flow, vascular stiffening, aneurysm formation, and vascular rupture and atherogenesis (Ungvari et al. 2007, 2010a, 2018b; Csiszar et al. 2002, 2007a, b, 2009; Toth et al. 2014, 2015a).
There is strong evidence that aging is associated with increased production of reactive oxygen species (ROS) in the vascular wall (Ungvari et al. 2018b). Studies on vascular cells isolated from nonhuman primates suggest that mitochondria (Ungvari et al. 2011a) are important sources of increased ROS production in aging. This conclusion is in accord with the findings of studies demonstrating elevated mitochondrial oxidative stress in isolated arteries and primary cells derived from aged rodents (Ungvari et al. 2007, 2008; Springo et al. 2015; Tarantini et al. 2018a). Additional mechanisms of age-related increases in free radical production include activation of NADPH oxidases (Ungvari et al. 2018b; Csiszar et al. 2002; van der Loo et al. 2000; Adler et al. 2003; Donato et al. 2007; Jacobson et al. 2007).
Perhaps more important than ROS production per se is the ability of the vascular cells to protect themselves and scavenge/eliminate excess ROS and repair or otherwise cope with the oxidative damage. The ability of the vascular tissue to respond to oxidative stress and return to homeostasis (“resilience”) has been shown to be progressively diminished as a function of age. As a consequence, in the aged vasculature the same pathological stressors elicit exacerbated oxidative stress as compared to young vessels (Toth et al. 2015a; Springo et al. 2015). It is believed that this age-related loss of vascular resilience, which impair redox homeostasis, significantly contributes to the increased propensity of the aged vasculature to pathological alterations ranging from endothelial dysfunction to atherosclerotic diseases and vascular structural damage (e.g., aorta aneurysma, cerebral microhemorrhages).
The Keap1-Nrf2-antioxidant response element pathway
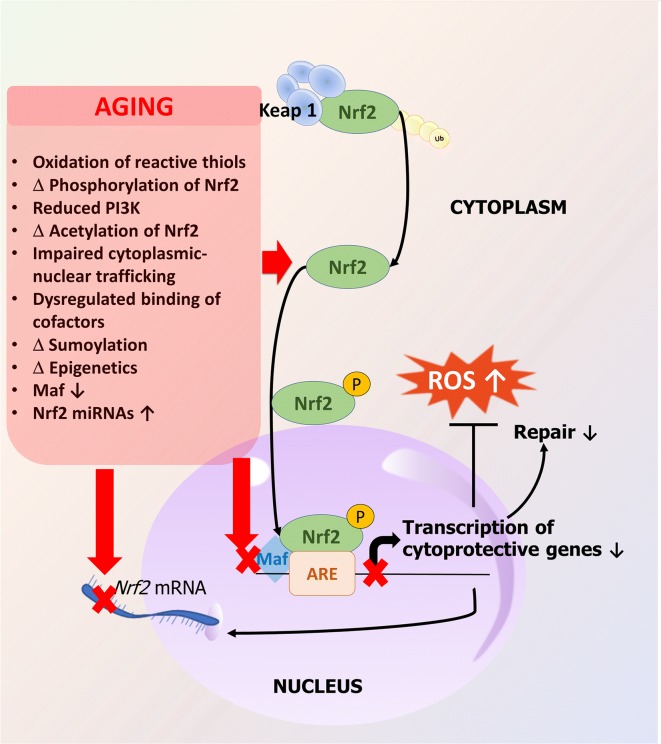
Among the adaptive stress response mechanisms responsible for cellular resilience, an age-related deterioration of Nrf2 (nuclear factor-erythroid-2-related factor 2)/ARE (antioxidant response element)-dependent antioxidative defense pathways is of particular importance (Ungvari et al. 2011a; Ahn et al. 2018; Bailey-Downs et al. 2012; Csiszar et al. 2012, 2014a; Fulop et al. 2018; Tarantini et al. 2018b). Nrf2 is an evolutionarily highly conserved transcription factor regulating the transcription of an array of cytoprotective (antioxidant, pro-survival, anti-inflammatory, and macromolecular damage repair) genes. Under basal conditions, Nrf2 is bound in the cytosol by its negative regulator Keap1 (Fig. 1). Keap1 is a substrate adapter protein for the Cullin3-Rbx1 E3 ligase complex and constitutively targets Nrf2 for proteasomal degradation. Oxidation of critical cysteine residues on Keap1 by increased levels of ROS or electrophiles causes modification in its tertiary structure, leading to dissociation of Nrf2 from Keap1. Keap1 then undergoes degradation, allowing Nrf2 to translocate to the nucleus where it binds to conserved ARE sequences in the promoter region of the Nrf2 target genes. DNA binding of Nrf2 requires small Maf proteins; thus, availability of Maf proteins contributes to the regulation of Nrf2 activity. Negative regulators of Nrf2 activation include Bach1, which competes with Nrf2 for binding to Maf proteins. The Keap1-Nrf2-ARE pathway regulates the expression of hundreds of genes that are involved in the cytoprotective response against oxidative stressors. Classical Nrf2 target genes encode antioxidant proteins (e.g., catalase, heme oxygenase 1, glutathione peroxidases, NAD(P)H quinone dehydrogenase (NQO1), glutathione-S-transferases, peroxiredoxins, thioredoxins, thioredoxin reductases, and glutamate-cysteine ligase, which is the rate-limiting enzyme for glutathione synthesis). Nrf2 also regulates expression of genes involved in autophagy and the proteasome.
Fig. 1.
Mechanisms involved in aging-induced Nrf2 dysfunction. The Nrf2 response pathway is an evolutionarily conserved adaptive mechanism that attenuates oxidative stress, limits the cellular and macromolecular damage caused by the increased free radical production, and maintains cellular homeostasis in the vascular wall. Nrf2 regulates the transcription of over 200 cytoprotective genes involved in antioxidant defenses and repair of macromolecular damage through the antioxidant response elements (ARE) present in target gene promoters. Aging is associated with dysregulation of Nrf2, and as a result, in the aged vasculature oxidative stress fails to activate Nrf2-regulated ROS detoxification systems and repair pathways. The scheme depicts potential mechanisms that contribute to the dysregulation of Nrf2 activation during the vascular aging process
Important for the present discussion, there is increasing evidence that Nrf2 regulates DNA repair pathways, limiting DNA damage induced by genotoxic and oxidative stressors (Jayakumar et al. 2015; Sekhar and Freeman 2015; Singh et al. 2013). The impact of Nrf2 on DNA repair is not dependent on its antioxidant functions. Instead, many repair genes that are involved in DNA repair, including homologous recombination repair and base excision repair mechanisms, are directly regulated by Nrf2 (Jayakumar et al. 2015). Chromatin immunoprecipitation (ChIP) assays also demonstrate that Nrf2 binds to the promoter of key enzymes in the base excision repair pathway and RNAi experiments show that Nrf2 dysfunction associates with impaired DNA repair (Singh et al. 2013). Genetic Nrf2 depletion in mice was shown to result in persistence of residual DNA damage induced by genotoxic stresses.
Mechanisms of aging-induced Nrf2 dysfunction
There is strong evidence that Nrf2 transcriptional activity declines with age in the vasculature (Ungvari et al. 2011a, b), which contributes to the development of a wide range of vascular aging phenotypes. Previous studies in laboratory rodents and nonhuman primates show that aging impairs the ability of vascular endothelial and smooth muscle cells to mount an effective Nrf2-dependent antioxidant defense in response to oxidative stressors, which results in more robust oxidative stress and oxidative damage in aged cells than in young cells (Ungvari et al. 2011a, b; Csiszar et al. 2012, 2014a). Interestingly, the role of Nrf2 dysfunction in the aging process may be evolutionarily conserved. Previous studies show that aging Drosophila progressively lose the ability to activate Nrf2 targets in response to acute stress exposure, which contributes to age-associated functional decline (Rahman et al. 2013). Further, the Nrf2 homolog SKN-1 was shown to regulate longevity in C. elegans (Tullet et al. 2008). Recent studies demonstrate that aging in C. elegans is also associated with significant impairment of SKN-1-mediated adaptive oxidative stress responses (Raynes et al. 2017).
The mechanisms underlying dysregulation of Nrf2-mediated antioxidant responses in aging are likely multifaceted. Expression of Nrf2 and Nrf2-driven antioxidant genes is modulated by microRNAs (Cheng et al. 2013). Studies on cultured endothelial cells derived from young and aged rats demonstrate that aging-induced dysregulation of miRNA expression contributes to downregulation of Nrf2 (Csiszar et al. 2014a). Aging may also impair the pathways that regulate Nrf2 activation (e.g., dysregulation in binding of cofactors) and nuclear translocation. Age-related alterations in post-translational protein modifications, including oxidation of sulfhydryl groups in cysteine residues in Keap1 and phosphorylation and/or acetylation of Nrf2, can interfere with release/activation of Nrf2. In addition, cytoskeletal alterations may impair cytoplasmic-nuclear trafficking. Aging may also dysregulate binding of cofactors, such as small Maf proteins, impairing ARE-specific DNA binding of Nrf2 and downregulating the transcription of Nrf2 target genes. Interestingly, studies in cells from patients with Hutchinson-Gilford progeria syndrome demonstrate that progerin sequesters Nrf2 and thereby causes its subnuclear mislocalization, resulting in impaired Nrf2 transcriptional activity (Kubben et al. 2016).
It is possible that circulating anti-geronic and pro-geronic factors contribute to the dysregulation of Nrf2 in aged vessels. In humans, aging results in a marked decline in circulating IGF-1, which is thought to promote age-related vascular pathologies, including atherosclerosis (Higashi et al. 2012), cerebral microhemorrhages (Tarantini et al. 2017a), and endothelial dysfunction (Toth et al. 2015b). Importantly, decreased circulating IGF-1 results in marked Nrf2 dysfunction in mouse arteries (Bailey-Downs et al. 2012). There is also evidence that soluble klotho, a putative anti-aging factor, exerts protective effects on vascular cells by inducing Nrf2 (Maltese et al. 2017).
Role of Nrf2 dysfunction in vascular endothelial cells: from vasomotor dysfunction and inflammation to BBB disruption and impaired angiogenesis
Nrf2/ARE-regulated pathways play multifaceted roles in endothelial physiology and pathophysiology, exerting antioxidative, anti-inflammatory, and cytoprotective effects. There is evidence that aging is associated with Nrf2 dysfunction in endothelial cells (Ungvari et al. 2011a, b), which likely has deleterious consequences for cardiovascular health span.
Studies in genetically modified mice demonstrate that increased oxidative stress due to Nrf2 deficiency impairs endothelial function, reducing functional hyperemia in the brain (Tarantini et al. 2018b). Genetic Nrf2 depletion also exacerbates endothelial dysfunction induced by obesity in the brain, aorta, and the skeletal muscle microcirculation (Tarantini et al. 2018b; Ungvari et al. 2010b, 2011c), mimicking the aging phenotype (Tucsek et al. 2014a). Increased oxidative stress due to Nrf2 dysfunction also promotes proinflammatory phenotypic alteration in endothelial cells, including activation of NF-κB. Adaptive Nrf2 activation has been observed in endothelial cells in response to hyperglycemia (Ungvari et al. 2011c) and high levels of advanced glycation end products (He et al. 2010), which likely protect the vasculature against the deleterious pro-oxidative and proinflammatory effects of diabetes mellitus. It is likely that age-related Nrf2 dysfunction contributes to the exacerbation of vascular dysfunction induced by metabolic diseases in aging (Tucsek et al. 2014a, b; Bailey-Downs et al. 2013). Other pathological conditions in which endothelial Nrf2 activation likely plays critical protective roles include sepsis (Holloway et al. 2016; Kim et al. 2012; Thimmulappa et al. 2006a, b). Studies in endothelial cells suggest that induction of Nrf2-regulated genes (e.g., heme oxygenase-1) in response to treatment with septic sera is impaired in aging (Tucsek et al. 2013) and that the exacerbated endothelial dysfunction at the level of the microcirculation likely contributes to multiple organ failure observed in aged animals (Coletta et al. 2014). There is strong evidence that Nrf2 can be activated by atheroprotective shear stress in endothelial cells, which confers important anti-inflammatory effects (Chen et al. 2003; Dai et al. 2007; Warabi et al. 2007; Zakkar et al. 2009). We posit that age-related impairment of mechanosensitive activation of Nrf2/ARE-mediated pathways promotes atherogenesis.
Cerebromicrovascular endothelial health determines the integrity of the blood-brain barrier (BBB), which is critical for normal brain function. Compromised BBB plays a critical role in the pathogenesis of a number of age-related diseases of the central nervous system, including neurodegeneration. There is evidence that activation of the Nrf2/ARE-dependent cytoprotective mechanisms protect the integrity of the BBB under conditions of pathological stressors, including metabolic diseases (Tarantini et al. 2018b), brain injury (Zhao et al. 2007), and sepsis (Li et al. 2018). Genetic depletion of Nrf2 was reported to exacerbate BBB disruption induced by metabolic stressors (Tarantini et al. 2018b), mimicking the aging phenotype (Tucsek et al. 2014b). Nrf2 is a promising pharmacological target for prevention of BBB disruption. Accordingly, previous studies demonstrate that sulforaphane-mediated activation of Nrf2 in the cerebral vasculature prevents BBB disruption protecting the brain against stroke-related neurological dysfunction (Alfieri et al. 2013).
Angiogenic capacity of endothelial cells is also regulated by Nrf2. There is evidence that in the absence of functional Nrf2 endothelial cells exhibit impaired proliferation, reduced cellular migration, and impaired the ability to form capillary-like structures (Valcarcel-Ares et al. 2012). In addition to regulating angiogenic processes, the known anti-apoptotic action of Nrf2/ARE-mediated pathways likely contributes importantly to the preservation of the structural integrity of newly formed capillaries. It is likely that age-related Nrf2 deficiency contributes to increased rate of endothelial apoptosis (Csiszar et al. 2004, 2007b; Ungvari et al. 2011b) and impaired angiogenesis, promoting microvascular rarefaction (Anversa et al. 1994; Riddle et al. 2003; Sonntag et al. 1997).
Role of Nrf2 dysfunction in vascular smooth muscle cells: from atherosclerosis to structural damage
In vascular smooth muscle cells (VSMCs), adaptive activation of Nrf2 was demonstrated in response to a wide range of stressors, including exposure to oxidized LDL (Anwar et al. 2005) and inflammatory cytokines (Churchman et al. 2009). Preclinical studies suggest that Nrf2 activation in VSMCs mediates potent antiatherosclerotic effects (Bozaykut et al. 2014).
Previous studies demonstrated age-associated alterations in the homeostatic role of Nrf2-driven free radical detoxification mechanisms in the VSMCs (Ungvari et al. 2011a), which likely promote the pathogenesis of multiple age-related diseases. The available data suggest that Nrf2 dysfunction in VSMCs is causally linked to NF-κΒ activation and chronic low-grade vascular inflammation in aging (Ungvari et al. 2011a). Age-related Nrf2 dysfunction may also contribute to the age-related exacerbation of hypertension-induced vascular oxidative stress (Toth et al. 2015a; Springo et al. 2015) and its pathophysiological consequences, including the increased propensity for microvascular injury in aging (Toth et al. 2015a).
Recent studies demonstrate that age-related exacerbation of the pro-oxidative effects of hypertension leads to activation of matrix metalloproteinases and pathological remodeling of the extracellular matrix in the vascular wall, promoting the pathogenesis of intracerebral hemorrhages (Toth et al. 2015a; Ungvari et al. 2017a, 2018c). It can be speculated that Nrf2 dysfunction may contribute to increased oxidative stress-related vascular pathologies in aging, including the genesis of cerebral microhemorrhages and aorta aneurysms. Further preclinical studies are evidently needed to experimentally test these ideas. Interestingly, recent evidence shows that treatment with the Nrf2 activator dimethyl fumarate reduces the formation and rupture of intracranial aneurysms (Pascale et al. 2019).
Role of Nrf2 dysfunction promoting cellular senescence: a novel mechanism contributing to vascular aging
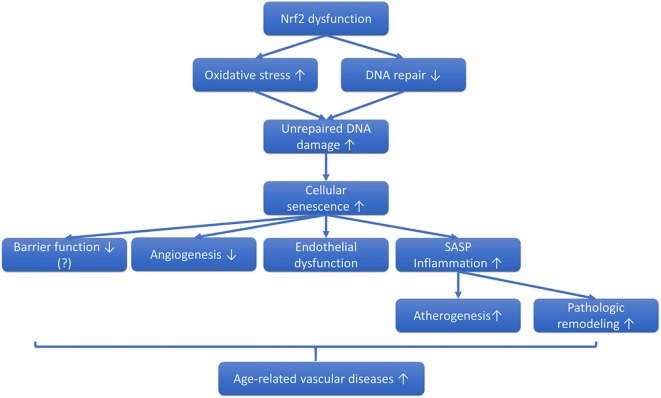
Among the cellular and molecular mechanisms contributing to organismal aging in recent years, cellular senescence has emerged as a fundamental aging process (Baker et al. 2016). There is also growing evidence that increased DNA damage (telomeric, non-telomeric, and/or mitochondrial DNA damage) plays a role in vascular aging by promoting cellular senescence (Fig. 2) (Bautista-Nino et al. 2016). Upon induction of cellular senescence, vascular cells undergo cell cycle arrest and therefore can no longer replicate, diminishing the repair and remodeling capacity of the vasculature. Senescent cells also acquire a highly inflammatory senescence-associated secretory phenotype, which consists of increased secretion of proinflammatory cytokines and chemokines, matrix metalloproteinases, and altered release of gaseotransmitters and eicosanoid mediators (Freund et al. 2010). There is growing evidence that increased presence of senescent vascular cells in the tissues contributes to the pathogenesis of various age-related diseases. Recent studies demonstrate that removal of senescent cells expressing the senescent marker cyclin-dependent kinase inhibitor p16INK4A in genetically modified mice (INK-ATTAC and p16-3MR mice) leads to a prolonged lifespan and general health span (Baker et al. 2011, 2016; Jeon et al. 2017; Abdul-Aziz et al. 2019; Kim et al. 2019; Patil et al. 2019; Farr et al. 2017; Xu et al. 2015) as well as improved cardiovascular health (Tarantini and Ungvari 2019, unpublished observation) (Roos et al. 2016), supporting a key role for cellular senescence in the process of aging.
Fig. 2.
Role of Nrf2 dysfunction in vascular senescence. The proposed model predicts that Nrf2 dysfunction exacerbates oxidative stress-induced DNA damage, by impairing both antioxidant defenses and DNA repair pathways in the vascular endothelial and smooth muscle cells. The unrepaired DNA damage triggers cellular senescence, which contributes to dysregulation of blood flow, atherogenesis, impaired angiogenesis, disruption of the microvascular barrier function, and pathological remodeling of both large arteries and the microcirculation. All of the aforementioned pathological processes contribute to the deterioration of vascular health in aging
Nrf2 activation has been shown to inhibit the induction of cellular senescence (Romero et al. 2019). Recent studies demonstrate that genetic depletion of the Nrf2 exacerbates age-related induction of cellular senescence in cerebral arteries, which contributes to upregulated vascular expression of inflammatory cytokines and a heightened inflammatory status of the hippocampus (Fulop et al. 2018). In addition, the effects of increased oxidative stress induced by cardiovascular risk factors (e.g., obesity) are also exacerbated in genetically Nrf2-deficient mice, at least in part, due to accelerated cellular senescence (Tarantini et al. 2018b; Ungvari et al. 2010b, 2011c).
Vasoprotection mediated through activation of Nrf2 by dietary interventions and pharmacological agents in aging
On the basis of the available experimental evidence, it has been proposed that the Nrf2/ARE-mediated antioxidative defense pathway may serve as a therapeutic target for neurovascular protection in stroke and other human disease conditions (Alfieri et al. 2011).
Previous studies show that age-related increase in oxidative stress in microvascular endothelial cells is prevented by the anti-aging dietary regimen caloric restriction (Csiszar et al. 2009, 2014a). Importantly, caloric restriction was shown to restore expression and transcriptional activity of Nrf2 to youthful levels in aged endothelial cells (Csiszar et al. 2014a), which likely significantly contribute to its antioxidative vasoprotective effects. Nrf2 activation was also shown to critically contribute to the anti-cancer effects of caloric restriction (Pearson et al. 2008a). The mechanisms by which caloric restriction upregulates Nrf2 likely includes downregulation of miRNAs that decrease mRNA expression in endothelial cells (Csiszar et al. 2014a).
To mimic the beneficial effects of caloric restriction and to develop novel interventions for vasoprotection in aging several pharmacological activators of Nrf2 have been identified and tested in preclinical studies. Resveratrol (3,4,5-trihydroxystilbene), a plant-derived polyphenolic compound, is a potent activator of Nrf2 in endothelial cells and vascular smooth muscle cells (Csiszar et al. 2012, 2014b; Ungvari et al. 2010b). Recent studies provide strong evidence that the treatment of laboratory rodents with resveratrol exerts significant vasoprotective effects both during aging and in pathological conditions associated with accelerated vascular aging (Toth et al. 2014, 2015a; Mattison et al. 2014; Oomen et al. 2009; Pearson et al. 2008b; Csiszar et al. 2008). In particular, resveratrol was shown to confer microvascular protection, increasing capillarization (Oomen et al. 2009), rescuing endothelium-mediated neurovascular coupling responses (Toth et al. 2014), and preventing hypertension-induced microvascular damage (Toth et al. 2015a) in the aged mouse brain. Previous studies suggest that long-term treatment with resveratrol also confers protection against cerebral vascular dysfunction during nutrient stress in nonhuman primates (Bernier et al. 2016). Resveratrol-induced endothelial protection is also manifested in the large vessels of rodent (Pearson et al. 2008b) and nonhuman primate models of aging (Mattison et al. 2014). It should be noted, however, that the effects of resveratrol are likely not specific to Nrf2 and involve activation of SIRT-1 as well.
Sulforaphane, which is an organosulfur compound found in cruciferous vegetables such as broccoli, cauliflower, and Brussels sprouts, is also a potent inducer of Nrf2 (Alfieri et al. 2013; Santin-Marquez et al. 2019). Importantly, sulforaphane was shown to inhibit vascular inflammation (Zakkar et al. 2009), prevent atherogenesis (Shehatou and Suddek 2016), and improve endothelial function (Pereira et al. 2017) in pathological conditions associated with accelerated vascular aging. Nrf2 activation also plays a critical role in endothelial protection mediated by estrogen and various phytoestrogens (Siow et al. 2007). Sulforaphane was shown to delay cellular senescence in vitro by attenuating cellular oxidative damage (Hariton et al. 2018). Other canonical Nrf2 activators, which have entered clinical trials in the USA with diverse indications, include the synthetic oleanane triterpenoid compound bardoxolone methyl (CDDO-Me), RTA 408, and dimethyl fumarate (Tecfidera). These compounds have shown to exert endothelial protection in preclinical studies. However, there is a paucity of data regarding their efficacy to improve vascular function in aging.
It should be noted that many electrophilic Nrf2 activators are present in natural products. In older adults, dietary supplementation with spices such as turmeric, as well as the inclusion of whole foods (e.g., broccoli sprouts and other cruciferous vegetables, green tea) that contain Nrf2 activator phytochemicals in their diet, is likely to exert important vasoprotective effects.
Conclusions
Nrf2 may provide a therapeutic target for countering vascular oxidative stress and inflammation associated with aging and pathological conditions characterized by accelerated vascular aging (Ungvari et al. 2017b, 2018c; Ashpole et al. 2017; Csiszar et al. 2017; Deepa et al. 2017; Grant et al. 2017; Tarantini et al. 2017b; Tucsek et al. 2017; Carlson et al. 2018; Cervellati et al. 2018; Chao et al. 2018; Csipo et al. 2018; Cunningham et al. 2018; Logan et al. 2018; Reglodi et al. 2018). In order to develop novel therapeutic interventions to promote cardiovascular and cerebrovascular health in older persons, it is essential to better understand the interconnected cellular pathways through which aging impairs Nrf2-dependent homeostatic mechanisms, reduces vascular resilience to stressors, and promotes cellular senescence. In addition to the Keap1-dependent mechanisms, future studies should also better characterize the role of age-related alterations in the autophagy lysosomal pathway in dysregulation of non-canonical activation of Nrf2 in the aging vasculature. There is strong evidence suggesting that cellular senescence plays an important role in vascular aging and that Nrf2 activation protects against induction of cellular senescence. Thus, future studies should also test the protective effects of long-term treatment with Nrf2 activators on senescence-related endpoints. Studies combining Nrf2 activators with senolytic drugs will be also very informative.
Acknowledgements
This work was supported by grants from the American Heart Association, the National Institute on Aging (R01-AG055395, R01-AG047879; R01-AG038747; P30-AG050911), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS056218, R01-NS100782), the Oklahoma Center for the Advancement of Science and Technology, and the Presbyterian Health Foundation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdul-Aziz AM, Sun Y, Hellmich C, Marlein CR, Mistry J, Forde E, Piddock RE, Shafat MS, Morfakis A, Mehta T, Di Palma F, Macaulay I, Ingham CJ, Haestier A, Collins A, Campisi J, Bowles KM, Rushworth SA. Acute myeloid leukemia induces protumoral p16INK4a-driven senescence in the bone marrow microenvironment. Blood. 2019;133:446–456. doi: 10.1182/blood-2018-04-845420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A, Messina E, Sherman B, Wang Z, Huang H, Linke A, Hintze TH. NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am J Physiol Heart Circ Physiol. 2003;285:H1015–H1022. doi: 10.1152/ajpheart.01047.2002. [DOI] [PubMed] [Google Scholar]
- Ahn B, Pharaoh G, Premkumar P, Huseman K, Ranjit R, Kinter M, Szweda L, Kiss T, Fulop G, Tarantini S, Csiszar A, Ungvari Z, Van Remmen H. Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol. 2018;17:47–58. doi: 10.1016/j.redox.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol. 2011;589:4125–4136. doi: 10.1113/jphysiol.2011.210294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri A, Srivastava S, Siow RC, Cash D, Modo M, Duchen MR, Fraser PA, Williams SC, Mann GE. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic Biol Med. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- Anversa P, Li P, Sonnenblick EH, Olivetti G. Effects of aging on quantitative structural properties of coronary vasculature and microvasculature in rats. Am J Phys. 1994;267:H1062–H1073. doi: 10.1152/ajpheart.1994.267.3.H1062. [DOI] [PubMed] [Google Scholar]
- Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med. 2005;39:227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci. 2012;67:313–329. doi: 10.1093/gerona/glr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. doi: 10.1093/gerona/gls238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Niño Paula, Portilla-Fernandez Eliana, Vaughan Douglas, Danser A., Roks Anton. DNA Damage: A Main Determinant of Vascular Aging. International Journal of Molecular Sciences. 2016;17(5):748. doi: 10.3390/ijms17050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Wahl D, Ali A, Allard J, Faulkner S, Wnorowski A, Sanghvi M, Moaddel R, Alfaras I, Mattison JA, Tarantini S, Tucsek Z, Ungvari Z, Csiszar A, Pearson KJ, de Cabo R. Resveratrol supplementation confers neuroprotection in cortical brain tissue of nonhuman primates fed a high-fat/sucrose diet. Aging (Albany NY) 2016;8:899–916. doi: 10.18632/aging.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozaykut P, Karademir B, Yazgan B, Sozen E, Siow RC, Mann GE, Ozer NK. Effects of vitamin E on peroxisome proliferator-activated receptor gamma and nuclear factor-erythroid 2-related factor 2 in hypercholesterolemia-induced atherosclerosis. Free Radic Biol Med. 2014;70:174–181. doi: 10.1016/j.freeradbiomed.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Carlson BW, Craft MA, Carlson JR, Razaq W, Deardeuff KK, Benbrook DM. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. Geroscience. 2018;40:325–336. doi: 10.1007/s11357-018-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervellati C, Trentini A, Bosi C, Valacchi G, Morieri ML, Zurlo A, Brombo G, Passaro A, Zuliani G. Low-grade systemic inflammation is associated with functional disability in elderly people affected by dementia. Geroscience. 2018;40:61–69. doi: 10.1007/s11357-018-0010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CT, Wang J, Wu HY, Huang JW, Chien KL. Age modifies the risk factor profiles for acute kidney injury among recently diagnosed type 2 diabetic patients: a population-based study. Geroscience. 2018;40:201–217. doi: 10.1007/s11357-018-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- Cheng X, Ku CH, Siow RC. Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic Biol Med. 2013;64:4–11. doi: 10.1016/j.freeradbiomed.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Churchman AT, Anwar AA, Li FY, Sato H, Ishii T, Mann GE, Siow RC. Transforming growth factor-beta1 elicits Nrf2-mediated antioxidant responses in aortic smooth muscle cells. J Cell Mol Med. 2009;13:2282–2292. doi: 10.1111/j.1582-4934.2009.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta C, Modis K, Olah G, Brunyanszki A, Herzig DS, Sherwood ER, Ungvari Z, Szabo C. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit Care. 2014;18:511. doi: 10.1186/s13054-014-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. Geroscience. 2018;40:337–346. doi: 10.1007/s11357-018-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar Anna, Ungvari Zoltan, Koller Akos, Edwards John G., Kaley Gabor. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiological Genomics. 2004;17(1):21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Orosz Z, Xiangmin Z, Buffenstein R, Ungvari Z. Vascular aging in the longest-living rodent, the naked mole rat. Am J Phys. 2007;293:H919–H927. doi: 10.1152/ajpheart.01287.2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Smith K, Rivera A, Orosz Z, Ungvari Z. Vasculoprotective effects of anti-TNFalfa treatment in aging. Am J Pathol. 2007;170:388–698. doi: 10.2353/ajpath.2007.060708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol. 2008;294:H2721–H2735. doi: 10.1152/ajpheart.00235.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Jimenez R, Pinto JT, Ballabh P, Losonczy G, Pearson KJ, de Cabo R, Ungvari Z. Anti-oxidative and anti-inflammatory vasoprotective effects of caloric restriction in aging: role of circulating factors and SIRT1. Mech Ageing Dev. 2009;130:518–527. doi: 10.1016/j.mad.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Sosnowska D, Wang M, Lakatta EG, Sonntag WE, Ungvari Z. Age-associated proinflammatory secretory phenotype in vascular smooth muscle cells from the non-human primate Macaca mulatta: reversal by resveratrol treatment. J Gerontol A Biol Sci Med Sci. 2012;67:811–820. doi: 10.1093/gerona/glr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–H306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Pinto JT, Gautam T, Kleusch C, Hoffmann B, Tucsek Z, Toth P, Sonntag WE, Ungvari Z (2014b) Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci [DOI] [PMC free article] [PubMed]
- Csiszar A, Tarantini S, Fulop GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham GM, Flores LC, Roman MG, Cheng C, Dube S, Allen C, Valentine JM, Hubbard GB, Bai Y, Saunders TL, Ikeno Y. Thioredoxin overexpression in both the cytosol and mitochondria accelerates age-related disease and shortens lifespan in male C57BL/6 mice. Geroscience. 2018;40:453–468. doi: 10.1007/s11357-018-0039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Bhaskaran S, Espinoza S, Brooks SV, McArdle A, Jackson MJ, Van Remmen H, Richardson A. A new mouse model of frailty: the Cu/Zn superoxide dismutase knockout mouse. Geroscience. 2017;39:187–198. doi: 10.1007/s11357-017-9975-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CD, Jafari N, Hou L, Li Y, Stewart JD, Zhang G, Lamichhane A, Manson JE, Baccarelli AA, Whitsel EA, Conneely KN. A longitudinal study of DNA methylation as a potential mediator of age-related diabetes risk. Geroscience. 2017;39:475–489. doi: 10.1007/s11357-017-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariton F, Xue M, Rabbani N, Fowler M, Thornalley PJ. Sulforaphane delays fibroblast senescence by curbing cellular glucose uptake, increased glycolysis, and oxidative damage. Oxidative Med Cell Longev. 2018;2018:5642148. doi: 10.1155/2018/5642148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE (2010) Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis. 2011 Apr;21(4):277–85. 10.1016/j.numecd.2009.12.008. [DOI] [PubMed]
- Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci. 2012;67:626–639. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway PM, Gillespie S, Becker F, Vital SA, Nguyen V, Alexander JS, Evans PC, Gavins FNE. Sulforaphane induces neurovascular protection against a systemic inflammatory challenge via both Nrf2-dependent and independent pathways. Vasc Pharmacol. 2016;85:29–38. doi: 10.1016/j.vph.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A, Yan C, Gao Q, Rincon-Skinner T, Rivera A, Edwards J, Huang A, Kaley G, Sun D. Aging enhances pressure-induced arterial superoxide formation. Am J Physiol Heart Circ Physiol. 2007;293:H1344–H1350. doi: 10.1152/ajpheart.00413.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar S, Pal D, Sandur SK. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat Res. 2015;779:33–45. doi: 10.1016/j.mrfmmm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi: 10.1038/nm.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Choi YK, Lee KS, Cho DH, Baek YY, Lee DK, Ha KS, Choe J, Won MH, Jeoung D, Lee H, Kwon YG, Kim YM. Functional dissection of Nrf2-dependent phase II genes in vascular inflammation and endotoxic injury using Keap1 siRNA. Free Radic Biol Med. 2012;53:629–640. doi: 10.1016/j.freeradbiomed.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Kim HN, Chang J, Iyer S, Han L, Campisi J, Manolagas SC, Zhou D, Almeida M. Elimination of senescent osteoclast progenitors has no effect on the age-associated loss of bone mass in mice. Aging Cell. 2019;18:e12923. doi: 10.1111/acel.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben N, Zhang W, Wang L, Voss TC, Yang J, Qu J, Liu GH, Misteli T. Repression of the antioxidant NRF2 pathway in premature aging. Cell. 2016;165:1361–1374. doi: 10.1016/j.cell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang P, Huang F, Jin J, Wu H, Zhang B, Wang Z, Shi H, Wu X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol Appl Pharmacol. 2018;340:58–66. doi: 10.1016/j.taap.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Logan S, Owen D, Chen S, Chen WJ, Ungvari Z, Farley J, Csiszar A, Sharpe A, Loos M, Koopmans B, Richardson A, Sonntag WE. Simultaneous assessment of cognitive function, circadian rhythm, and spontaneous activity in aging mice. Geroscience. 2018;40:123–137. doi: 10.1007/s11357-018-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese G, Psefteli PM, Rizzo B, Srivastava S, Gnudi L, Mann GE, Siow RC. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med. 2017;21:621–627. doi: 10.1111/jcmm.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, An SS, Santhanam L, Martin B, Faulkner S, Morrell C, Baur JA, Peshkin L, Sosnowska D, Csiszar A, Herbert RL, Tilmont EM, Ungvari Z, Pearson KJ, Lakatta EG, de Cabo R. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–190. doi: 10.1016/j.cmet.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale CL, Martinez AN, Carr C, Sawyer DM, Ribeiro-Alves M, Chen M, O'Donnell DB, Guidry JJ, Amenta PS, Dumont AS (2019) Treatment with dimethyl fumarate reduces the formation and rupture of intracranial aneurysms: role of Nrf2 activation. J Cereb Blood Flow Metab. 2019 Jun 20:271678X19858888. 10.1177/0271678X19858888. [DOI] [PMC free article] [PubMed]
- Patil P, Dong Q, Wang D, Chang J, Wiley C, Demaria M, Lee J, Kang J, Niedernhofer LJ, Robbins PD, Sowa G, Campisi J, Zhou D, Vo N. Systemic clearance of p16(INK4a) -positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell. 2019;18:e12927. doi: 10.1111/acel.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A. 2008;105:2325–2330. doi: 10.1073/pnas.0712162105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A, Fernandes R, Crisostomo J, Seica RM, Sena CM. The sulforaphane and pyridoxamine supplementation normalize endothelial dysfunction associated with type 2 diabetes. Sci Rep. 2017;7:14357. doi: 10.1038/s41598-017-14733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Sykiotis GP, Nishimura M, Bodmer R, Bohmann D. Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell. 2013;12:554–562. doi: 10.1111/acel.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes R, Juarez C, Pomatto LC, Sieburth D, Davies KJ. Aging and SKN-1-dependent loss of 20S proteasome adaptation to oxidative stress in C. elegans. J Gerontol A Biol Sci Med Sci. 2017;72:143–151. doi: 10.1093/gerona/glw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Atlasz T, Szabo E, Jungling A, Tamas A, Juhasz T, Fulop BD, Bardosi A. PACAP deficiency as a model of aging. Geroscience. 2018;40:437–452. doi: 10.1007/s11357-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev. 2003;2:149–168. doi: 10.1016/s1568-1637(02)00064-8. [DOI] [PubMed] [Google Scholar]
- Romero A, San Hipolito-Luengo A, Villalobos LA, Vallejo S, Valencia I, Michalska P, Pajuelo-Lozano N, Sanchez-Perez I, Leon R, Bartha JL, Sanz MJ, Erusalimsky JD, Sanchez-Ferrer CF, Romacho T, Peiro C. The angiotensin-(1-7)/mas receptor axis protects from endothelial cell senescence via klotho and Nrf2 activation. Aging Cell. 2019;18:e12913. doi: 10.1111/acel.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santín-Márquez Roberto, Alarcón-Aguilar Adriana, López-Diazguerrero Norma Edith, Chondrogianni Niki, Königsberg Mina. Sulforaphane - role in aging and neurodegeneration. GeroScience. 2019;41(5):655–670. doi: 10.1007/s11357-019-00061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar KR, Freeman ML. Nrf2 promotes survival following exposure to ionizing radiation. Free Radic Biol Med. 2015;88:268–274. doi: 10.1016/j.freeradbiomed.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehatou GS, Suddek GM. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp Biol Med (Maywood) 2016;241:426–436. doi: 10.1177/1535370215609695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B, Chatterjee A, Ronghe AM, Bhat NK, Bhat HK. Antioxidant-mediated up-regulation of OGG1 via NRF2 induction is associated with inhibition of oxidative DNA damage in estrogen-induced breast cancer. BMC Cancer. 2013;13:253. doi: 10.1186/1471-2407-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow RC, Li FY, Rowlands DJ, de Winter P, Mann GE. Cardiovascular targets for estrogens and phytoestrogens: transcriptional regulation of nitric oxide synthase and antioxidant defense genes. Free Radic Biol Med. 2007;42:909–925. doi: 10.1016/j.freeradbiomed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Springo Z, Tarantini S, Toth P, Tucsek Z, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates pressure-induced mitochondrial oxidative stress in mouse cerebral arteries. J Gerontol A Biol Sci Med Sci. 2015;70:1355–1359. doi: 10.1093/gerona/glu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, Csiszar A, Ungvari Z. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell. 2017;16:469–479. doi: 10.1111/acel.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zolei-Szenasi D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer’s disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares MN, Yabluchanskiy A, Tucsek Z, Hertelendy P, Kiss T, Gautam T, Zhang XA, Sonntag WE, de Cabo R, Farkas E, Elliott ME, Kinter MT, Deak F, Ungvari Z, Csiszar A. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood brain barrier disruption, neuroinflammation, amyloidogenic gene expression and cognitive decline in mice, mimicking the aging phenotype. J Gerontol A Biol Sci Med Sci. 2018;73:853–863. doi: 10.1093/gerona/glx177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT, Sporn MB, Yamamoto M, Kensler TW, Biswal S. Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun. 2006;351:883–889. doi: 10.1016/j.bbrc.2006.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari ZI. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Springo Z, Tucsek Z, Gautam T, Giles CB, Wren JD, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Aging exacerbates hypertension-induced cerebral microhemorrhages in mice: role of resveratrol treatment in vasoprotection. Aging Cell. 2015;14:400–408. doi: 10.1111/acel.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Gautam T, Sonntag WE, Toth P, Saito H, Salomao R, Szabo C, Csiszar A, Ungvari Z. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci. 2013;68:652–660. doi: 10.1093/gerona/gls232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Noa Valcarcel-Ares M, Tarantini S, Yabluchanskiy A, Fulop G, Gautam T, Orock A, Csiszar A, Deak F, Ungvari Z. Hypertension-induced synapse loss and impairment in synaptic plasticity in the mouse hippocampus mimics the aging phenotype: implications for the pathogenesis of vascular cognitive impairment. Geroscience. 2017;39:385–406. doi: 10.1007/s11357-017-9981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- Ungvari ZI, Labinskyy N, Gupte SA, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am J Physiol Heart Circ Physiol. 2008;294:H2121–H2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta E, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction and NF-kB activation in the non-human primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of Nrf2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari ZI, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson KJ, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kirkpatrick AC, Csiszar A, Prodan CI. Cerebral microhemorrhages: mechanisms, consequences, and prevention. Am J Physiol Heart Circ Physiol. 2017;312:H1128–H1143. doi: 10.1152/ajpheart.00780.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fulop GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. Geroscience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, Murfee WL, Pacher P, Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol. 2018;15:555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Yabluchanskiy A, Tarantini S, Toth P, Kirkpatrick AC, Csiszar A, Prodan CI. Repeated Valsalva maneuvers promote symptomatic manifestations of cerebral microhemorrhages: implications for the pathogenesis of vascular cognitive impairment in older adults. Geroscience. 2018;40:485–496. doi: 10.1007/s11357-018-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. doi: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL (2015) Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015 Dec 19;4:e12997. 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed]
- Zakkar Mustafa, Van der Heiden Kim, Luong Le Anh, Chaudhury Hera, Cuhlmann Simon, Hamdulay Shahir S., Krams Rob, Edirisinghe Indika, Rahman Irfan, Carlsen Harald, Haskard Dorian O., Mason Justin C., Evans Paul C. Activation of Nrf2 in Endothelial Cells Protects Arteries From Exhibiting a Proinflammatory State. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29(11):1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]