Abstract
Encephalopathy is an important complication associated with influenza, most frequently observed in young children, with a wide range of severity. The most severe category of influenza-associated encephalopathy (IAE) is acute necrotizing encephalopathy (ANE), characterized by high frequency of neurologic sequelae and fatal outcomes. We report two young siblings who developed fever and seizures with altered mental status. Influenza A(H1N1)pdm09 virus infection was identified in upper respiratory tract specimens from both patients, and neuroimaging revealed bilateral inflammatory lesions, consistent with acute necrotizing encephalopathy. Neither child had received influenza vaccination. Both children progressed to critical illness and required invasive mechanical ventilation. In addition to critical care management, both patients received high-dose corticosteroids, mannitol, anticonvulsants, and antiviral treatment of influenza. The older child recovered fully and was discharged 2 weeks after illness onset, but the younger sibling developed severe brainstem edema and cerebellar tonsillar herniation, and died on illness day 11. Both children tested positive for Ran Binding Protein 2 (RANBP2) gene mutations. RANBP2 is a genetic polymorphism associated with recurrent episodes of necrotizing encephalitis with respiratory viral infections. Annual influenza vaccination is especially important for ANE survivors, with or without RANBP2 mutations, their household contacts, and caregivers. During influenza season, close monitoring of any child with a history of neurological complications associated with respiratory illness is indicated, with prompt initiation of antiviral treatment with onset of acute respiratory illness, and influenza testing performed by molecular assay.
Keywords: acute necrotizing encephalitis, influenza A, influenza A H1N1, influenza-associated encephalopathy, RAN-binding protein 2
Encephalopathy is a severe influenza-associated complication and has been described most frequently in young children, particularly in Japan [1–3]. The onset of influenza-associated encephalopathy (IAE) typically occurs within a few days of the onset of influenza signs and symptoms, and the outcomes range from full recovery to neurologic sequelae in survivors or to fulminant progression and brain death [1–3]. IAE is distinct from Reye syndrome and not associated with salicylate exposure; although the transaminase level can be elevated, hypoglycemia and hyperammonemia are uncommon [1, 2]. Acute necrotizing encephalopathy (ANE) of childhood, the most severe form of IAE, first described in Japan in 1995, is characterized by sudden onset of fever, convulsions, coma, and sometimes death; symmetric inflammatory brain lesions are noted on neuroimaging [4].
IAE has been reportedly associated with influenza A, B, and C virus infections of the respiratory tract that can trigger a dys-regulated host cytokine response [5–9]. Before the 2009 H1N1 pandemic, sporadic cases of IAE, including ANE with fatal outcome, were reported infrequently in the United States [10–13]. During the 2009 pandemic, a large increase in pediatric IAE cases associated with influenza A(H1N1)pdm09 virus infection was noted in Japan [3]. Sporadic cases of influenza A(H1N1) pdm09–associated IAE in children have been reported in the United States and worldwide [14–17]. Here, we report cases of IAE with ANE that occurred concurrently in 2 young siblings, from nonconsanguineous parentage, who were heterozygous for a missense mutation in the nuclear pore protein Ran-binding protein 2 gene (RANBP2); mutations in RANBP2 have been linked to infection-triggered familial and recurrent ANE [18]
CASE REPORTS
Case 1
A 5-year-old boy in Mexico with a history of complex febrile seizures since the age of 2 years developed fever up to 39.4°C and cough in January 2017, and he received outpatient treatment with oral antibiotics for pharyngitis and ibuprofen for fever. He experienced nausea, emesis, and nonbloody diarrhea over the following 2 days. On illness day 4, he developed seizures and altered mental status and was admitted to the pediatric intensive care unit (PICU) of hospital A. Two prolonged generalized tonic-clonic seizures were observed, and anticonvulsant therapy with intravenous (IV) levetiracetam and IV midazolam was started. Electroencephalography results were reportedly abnormal. Computed tomography (CT) of his head revealed cerebral edema. Brain magnetic resonance imaging (MRI), performed 2 days later, revealed bilateral temporal lobe lesions with sub-cortical white matter lesions, consistent with encephalitis. Mannitol and methylprednisolone (1 g/24 hours) were administered. A lumbar puncture yielded no white blood cells, and Gram-staining results were negative. An upper respiratory tract specimen tested positive for influenza A viral antigen, and oral oseltamivir treatment was started on illness day 4. Other admission laboratory testing results are listed in Table 1. The patient had received all recommended routine childhood immunizations except for the influenza vaccine.
Table 1.
Laboratory Results for Case 1 (5-Year-Old Boy)
| Test Results | |||
|---|---|---|---|
| Testa | Hospital A, Illness Day 4 | Hospital B, Illness Day 8 (Weight, 23 kg; IV Fluid: NS) |
Hospital C, Illness Day 8 (Weight, 23 kg; IV Fluids, D5 1/4NS and 2 mEq KCI/L) |
| Blood tests | |||
| Sodium (mmol/L) | 144 | 143 | 144 |
| Potassium (mmol/L) | 3.5 | 4.2 | 3.3 |
| Chloride (mmol/L) | 105 | 111 | 110 |
| Calcium (mg/dL) | 9.7 | 8.1 | 7.9 |
| Indirect bilirubin (mg/dL) | 0.20 | — | — |
| Direct bilirubm (mg/dL) | 0.10 | — | — |
| Total bilirubin (mg/dL) | 0.30 | 0.1 | 0.2 |
| ALT (U/L) | 42.0 | 23 | 20 |
| AST (U/L) | 57.0 | 30 | 32 |
| GGT (U/L) | 19.0 | — | — |
| Albumin (g/dL) | 4.5 | 2.7 | 2.5 |
| Protein (g/dL) | 7.9 | 6.0 | 5.5 |
| Glucose (mg/dL) | 148 | 89 | 98 |
| Creatinine (mg/dL) | 0.3 | 0.25 | 0.27 |
| BUN (mg/dL) | 7 94 | 16 | 17 |
| Alkaline phosphatase (U/L) | 208.0 | 147 | 123 |
| Phosphorus (mg/dL) | 6.5 | — | — |
| Ammonia (μmol/L) | — | — | 56 |
| WBC (×103/μL) | 4.67 | 8.0 | 7.5 |
| HgB (g/dL) | 13.1 | 12.7 | 12.4 |
| HCT (%) | 39 2 | 37 4 | 35.8 |
| Platelets (×103/μL) | 253 | 182 | 161 |
| Neutrophils (%) | 57 | 73.4 | 70 |
| Bands (%) | 0 | — | 7 |
| Lymphocytes | |||
| Rel (%) | 32 | 17.7 | 22 |
| Total (x103/μL) | 67.6 | 1.41 | — |
| Monocytes(%) | 11 | 85 | — |
| Eosmophils (%) | 0 | 0 | — |
| Basophils (%) | 0 | 0.1 | — |
| Sedimentation rate (mm/hour) | — | 6 | — |
| Lactic acid (mmol/L) | — | 0.7 | — |
| PT (s) | — | — | 14.0 |
| PTT (s) | — | — | |
| INR | — | — | 1.1 |
| Fibrinogen (mg/dL) | — | — | 261 |
| Urinalyses | |||
| Hematuria | Negative | Negative | Negative |
| Proteinuria | Trace | Negative | Negative |
| Microbiology tests | |||
| Respiratory pathogen testing | Upper respiratory specimen: influenza A Ag, positive ; influenza B Ag, negative | Nasopharyngeal wash specimen: influenza A Ag, negative; influenza B Ag, negative | BioFire respiratory panel PCR, nasal swab: influenza A H1N1 2009, positive; influenza A H1, positive; influenza A, positive |
| Blood culture | — | Negative | |
| Urine culture | — | Negative | Negative |
| Respiratory culture of ET tube | — | — | Negative |
| Other stool testing | — | Clostridium difficile tox amp, negative | — |
| Cerebral spinal fluid | |||
| Color | Clear | — | — |
| Glucose (mg/dL) | 110 | — | — |
| Protein (mg/dL) | 2.0 | — | — |
| Cells/mL | 0 | — | — |
Abbreviations: Ag, antigen; ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; D5, 5% dextrose; ET, endotracheal; GGT, γ-glutamyl transferase; HCT, hematocrit; HgB, hemoglobin; INR, international normalized ratio; IV, intravenous; NS, normal saline; PT, prothrombin time; PTT, partial thromboplastin time; tox amp, toxin amplification assay; WBC, white blood cells.
Laboratory results were obtained from testing at hospital admission or earliest available testing.
On illness day 8, the patient was transferred to the emergency department of hospital B, where he was noted to have a Glasgow Coma Scale score of 7; he was placed on invasive mechanical ventilation. Radiography of his chest revealed bilateral infiltrates. A head CT scan revealed bilateral decreased attenuation involving the capsule area. Later that day, he was transferred to the PICU of hospital C, where a neurological examination revealed intact pupillary light and gag reflex. Inotropic support was required for his bradycardia and occasional hypotension. He was started on IV vancomycin, IV ceftriaxone, and oral oseltamivir. His hepatic transaminase levels and results of his coagulation studies were all within their reference range (Table 1). His initial white blood cell count was 7500/μL with 70% neutrophils and 22% lymphocytes. A repeat head CT scan performed after his admission to hospital C revealed symmetric areas of decreased attenuation in the external capsules and inferior temporal lobes bilaterally, with partial effacement of the cortical sulci. Electroencephalography revealed bilateral parietal spike activity. A nasopharyngeal swab tested positive for influenza A(H1N1)pdm09 viral RNA in a multiplex respiratory pathogen panel (BioFire Diagnostics, Salt Lake City, Utah). Results of brain MRI performed on illness day 9 were significant for bilaterally symmetric restricted diffusion and extensive diffuse signal abnormalities along the external capsules, medial temporal lobes, and pons, consistent with ischemia/infarction (Figure 1). Levetiracetam was continued for the necrotizing encephalitis diagnosis. Oral oseltamivir was discontinued, and IV peramivir was started. Antibiotics were discontinued, and he received 1 dose of IV immunoglobulin (1 g/kg) and was started on methylprednisolone (30 mg/kg per day); after 4 days, he was switched to prednisolone (2 mg/kg per day) for an additional 3 days. The results of bacterial cultures of blood, urine, and tracheal aspirate specimens obtained on admission were negative.
Figure 1.
T2-weighted sagittal magnetic resonance image showing bilateral enhancement of the external capsules in a 5-year-old boy with acute necrotizing encephalopathy.
His neurological status improved, and he was extubated on illness day 10 and transferred to the general inpatient floor on room air for additional monitoring and physical therapy and to complete a 5-day course of IV peramivir. On illness day 14, his neurological examination was normal, quadrivalent inactivated influenza vaccine was administered, and the patient was discharged to his home on levetiracetam anticonvulsant therapy. Blood obtained for genetic testing (Fulgent Diagnostics, Temple City, California) revealed a missense mutation (c.1754C→T p.Thr585MET) heterozygous for RANBP2.
Case 2
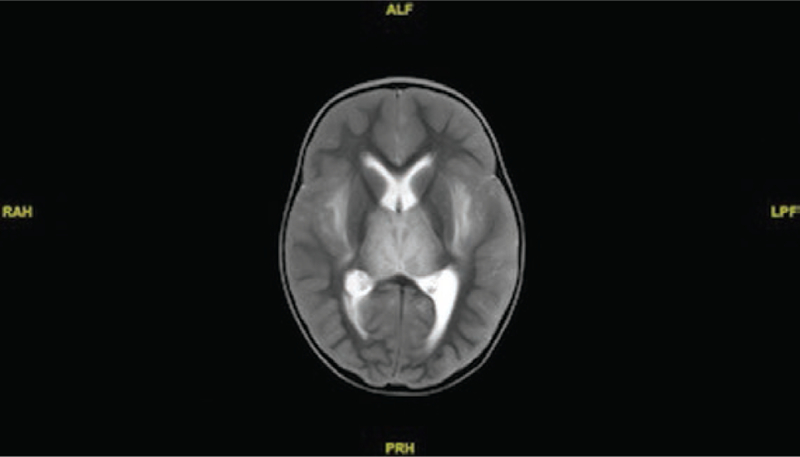
A previously healthy 17-month-old girl, the sister of the boy described in case 1, had fever onset of 40°C, eye deviation, and several episodes of focal seizure-like activity in January 2017. The patient had received all recommended routine childhood immunizations except for the influenza vaccine. Her parents denied that the child had any antecedent respiratory symptoms. She was admitted to hospital A on the same day as her brother and was transferred to the PICU with a clinical diagnosis of encephalitis and influenza, despite negative influenza antigen testing results on an upper respiratory specimen; she was started on empiric oseltamivir treatment. Diarrhea and vomiting were noted. Twelve hours after admission, her seizures reoccurred and IV levetiracetam was administered. Brain MRI revealed multiple hypodense lesions in the limbic region, hippocampus, and frontal lobe with severe brainstem edema. Methylprednisolone (1 g/24 hours) and mannitol were administered. The patient was intubated for respiratory failure with associated anisocoria and decerebrate posturing.
On illness day 5, the patient was transferred to hospital B and then transferred to hospital C later that day. The physical examination on admission was significant for mechanical ventilation without sedation and absent pupillary, corneal, and gag reflexes, but withdrawal to pain was noted. A head CT scan revealed diffuse effacement of the sulci and basilar cisterns. A chest CT scan revealed atelectasis or infiltrate in the left lingula. Empiric acyclovir, oseltamivir, vancomycin, and ceftriaxone were administered. Laboratory study results were remarkable for a serum sodium level of 170 mmol/L (Table 2). Immediate management of diabetes insipidus with vasopressin was started, and levetiracetam was continued for seizure management. A nasopharyngeal swab tested positive for influenza A(H1N1)pdm09 viral RNA via a multiplex respiratory pathogen panel (BioFire Diagnostics), and peramivir was continued in place of oseltamivir. Brain MRI results were markedly abnormal; severe brainstem edema and cerebellar tonsillar herniation, bilateral severe hemorrhagic and edematous thalami, bilateral signal abnormalities in the external capsules and medial temporal lobes, and increased signal in the left parietal lobe were seen (Figures 2 and 3). The results of bacterial cultures of blood, urine, and tracheal aspirate specimens obtained on admission were negative. The child died on illness day 11. Genetic testing of her blood revealed a missense mutation (c.1754C→T p.Thr585MET) heterozygous for RANBP2.
Table 2.
Laboratory Results for Case 2 (17-Month-Old Girl)
| Test Results | |||
|---|---|---|---|
| Testa | Hospital A, Illness Day 1 | Hospital B, Illness Day 5 (Weight, 12 kg; IV Fluid: NS) |
Hospital C, Illness Day 5 (Weight, 9.1 kg; IV Fluids: D5 1/4NS + 2 mEq KCl/L) |
| Blood test | |||
| Sodium (mmol/L) | 135.0 | 155 | 170 |
| Potassium (mmol/L) | 4.3 | 3.9 | 2.8 |
| Chloride (mmol/L) | 101.0 | 124 | 134 |
| Calcium (mg/dL) | 9.6 | 7.4 | 7.6 |
| Indirect bilirubin (mg/dL)) | 0.20 | — | — |
| Direct bilirubin (mg/dL) | 0.10 | — | — |
| Total bilirubin (mg/dL) | 0.30 | 0.1 | 0.1 |
| ALT (U/L) | 32 | 28 | 49 |
| AST (U/L) | 43 | 49 | 26 |
| GGT (U/L) | 14.0 | — | — |
| Albumin (g/dL) | 4.4 | 2.8 | 2.6 |
| Protein (g/dL) | 7.0 | 502 | 5.1 |
| Glucose (mg/dL) | 121.0 | 227 | 123 |
| Creatinine (mg/dL) | 0.2 | 0018 | 0.15 |
| BUN (mg/dL) | 10.28 | 6 | 6 |
| Alkaline phosphatase (U/L) | 202 | 179 | 164 |
| Phosphorus (mg/dL) | 4.8 | — | — |
| Ammonia (μmol/L) | — | — | 64 |
| WBC(×103/μL) | 3.81 | 5.4 | 4.6 |
| HgB (g/dL) | 10.6 | 10.8 | 10.7 |
| HCT (%) | 31.8 | 31.6 | 31.6 |
| Platelets (×103/μL) | 229 | 180 | 202 |
| Neutrophils(%) | 65.0 | 65 | 43 |
| Bands (%) | 3.0 | — | 11 |
| Lymphocytes | |||
| Rel (%) | 26.0 | 26.1 | 40 |
| Total (×103/μL) | 7.53 | — | — |
| Monocytes (%) | 5.0 | 7.4 | 3 |
| Eosmophils (%) | 1.0 | 0 | — |
| Basophils (%) | 0 | 0 | — |
| Sedimentation rate (mm/hour) | — | 1 | — |
| Lactic acid (mmol/L) | — | 0.8 | — |
| Urinalysis | |||
| Hematuria (per hpf) | — | Negative | 6 |
| Proteinuria | — | Negative | Negative |
| Microbiology tests | |||
| Respiratory pathogen testing | Upper respiratory specimen: influenza A Ag, negative; influenza B Ag, negative; upper respiratory specimen: influenza A Ag, positive; influenza B antigen, negative | NP wash: influenza A Ag, positive; influenza B Ag, negative | BioFire respiratory panel PCR, nasal swab: influenza A H1N1 2009, positive; influenza A H1, positive; influenza A, positive |
| Blood culture | — | Negative | Negative |
| Urine culture | — | Negative | Negative |
| Respiratory culture, ET tube | — | — | Negative |
| Other stool testing | Rotavirus and adenovirus stool Ags, negative | — | — |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; BUN, blood urea nitrogen; D5, 5% dextrose; ET, endotracheal; GGT, γ-glutamyl transferase; HCT, hematocrit; HgB, hemoglobin; hpf, high-power field; IV, intravenous; NP, nasopharyngeal; NS, normal saline; PCR, polymerase chain reaction; WBC, white blood cells.
Laboratory results were obtained from testing at hospital admission or earliest available testing.
Figure 2.
T2-weighted sagittal magnetic resonance image showing enhancement of the bilateral thalami and external capsule in a 17-month-old girl with necrotizing encephalitis.
Figure 3.
T2-weighted coronal magnetic resonance image showing enhancement and edema of the brainstem in a 17-month-old girl with necrotizing encephalopathy.
DISCUSSION
We report here the cases of 2 siblings with severe concurrent influenza A(H1N1)pdm09 virus–associated ANE, including 1 surviving child without typical thalamic lesions on neuroimaging. Neither sibling had a history of salicylate exposure or laboratory findings consistent with Reye syndrome. Although reports of IAE have been reported worldwide, to our knowledge, only 3 previous fatal cases of ANE with influenza A(H1N1)pdm09 virus infection in the United States have been reported [17, 19, 20]. The pathogenesis of ANE is thought to involve cytokine dysregulation, which can result in systemic inflammation, multiorgan injury, vascular leak, brain cell apoptosis, and cerebral edema along with a high mortality rate or a large proportion of survivors with neurologic sequelae, particularly in young children [21]. In a study of 184 patients with IAE aged <15 years in Japan, independent factors associated with death were an AST level of ≥500 U/L, a glucose level of ≥150 mg/dL, hematuria or proteinuria (as seen with urinalysis), and administration of diclofenac sodium [22]. The patient in case 1 did not have any of these poor prognostic indicators and recovered, but the patient in case 2 had hyperglycemia and hematuria, and she died.
Both siblings tested positive for a RANBP2 gene mutation. Identification of a familial autosomal dominant ANE with RANBP2 gene mutations in 19 families worldwide has been reported; these mutations are characterized by recurrent rapid onset of ANE triggered by viral infections [23]. Other genetic factors have been implicated in patients with ANE and acute encephalopathy with biphasic seizures and late reduced diffusion [21]. The patient in case 1 had a history of complex febrile seizures triggered by suspected influenza at the age of 2 years. A small study in which 10 patients with ANE and RANBP2 mutations and 9 patients with familial or recurrent ANE without RANBP2 mutations were compared found that patients in the latter group had a worse outcome [24]. Although our findings support recommendations for RANBP2 gene mutation testing in ANE survivors [24–26], annual influenza vaccination is recommended for all children aged ≥6 months in the United States and might be especially important in ANE survivors with or without a RANBP2 mutation, their household contacts, and their care-givers [27]. Given the potential for ANE recurrence [24–26], close monitoring of children with a history of neurological complications associated with respiratory illness is indicated during influenza season along with prompt initiation of antiviral treatment with the onset of respiratory illness and influenza testing via molecular assay.
Footnotes
Disclaimer. The views expressed are those of the authors and do not necessarily represent the official policy of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Togashi T, Matsuzono Y, Narita M, Morishima T. Influenza-associated acute encephalopathy in Japanese children in 1994–2002. Virus Res. 2004; 103:75–8. [DOI] [PubMed] [Google Scholar]
- 2.Morishima T, Togashi T, Yokota S, et al. ; Collaborative Study Group on Influenza-Associated Encephalopathy in Japan. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis 2002; 35:512–7. [DOI] [PubMed] [Google Scholar]
- 3.Gu Y, Shimada T, Yasui Y, et al. National surveillance of influenza-associated encephalopathy in Japan over six years, before and during the 2009–2010 influenza pandemic. PLoS One 2013; 8:e54786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry 1995; 58:555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amin R, Ford-Jones E, Richardson SE, et al. Acute childhood encephalitis and encephalopathy associated with influenza: a prospective 11-year review. Pediatr Infect Dis J 2008; 27:390–5. [DOI] [PubMed] [Google Scholar]
- 6.Newland JG, Romero JR, Varman M, et al. Encephalitis associated with influenza B virus infection in 2 children and a review of the literature. Clin Infect Dis 2003; 36:e87–95. [DOI] [PubMed] [Google Scholar]
- 7.Takayanagi M, Umehara N, Watanabe H, et al. Acute encephalopathy associated with influenza C virus infection. Pediatr Infect Dis J 2009; 28:554. [DOI] [PubMed] [Google Scholar]
- 8.Lin CH, Huang YC, Chiu CH, et al. Neurologic manifestations in children with influenza B virus infection. Pediatr Infect Dis J 2006; 25:1081–3. [DOI] [PubMed] [Google Scholar]
- 9.Kawada J, Kimura H, Ito Y, et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J Infect Dis 2003; 188:690–8. [DOI] [PubMed] [Google Scholar]
- 10.Maricich SM, Neul JL, Lotze TE, et al. Neurologic complications associated with influenza A in children during the 2003–2004 influenza season in Houston, Texas. Pediatrics 2004; 114:e626–33. [DOI] [PubMed] [Google Scholar]
- 11.Weitkamp JH, Spring MD, Brogan T, et al. Influenza A virus-associated acute necrotizing encephalopathy in the United States. Pediatr Infect Dis J 2004; 23:259–63. [DOI] [PubMed] [Google Scholar]
- 12.Bhat N, Wright JG, Broder KR, et al. ; Influenza Special Investigations Team. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med 2005; 353:2559–67. [DOI] [PubMed] [Google Scholar]
- 13.Newland JG, Laurich VM, Rosenquist AW, et al. Neurologic complications in children hospitalized with influenza: characteristics, incidence, and risk factors. J Pediatr 2007; 150:306–10. [DOI] [PubMed] [Google Scholar]
- 14.Baltagi SA, Shoykhet M, Felmet K, et al. Neurological sequelae of 2009 influenza A (H1N1) in children: a case series observed during a pandemic. Pediatr Crit Care Med 2010; 11:179–84. [DOI] [PubMed] [Google Scholar]
- 15.Surana P, Tang S, McDougall M, et al. Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur J Pediatr 2011; 170:1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farooq O, Faden HS, Cohen ME, et al. Neurologic complications of 2009 influenza-A H1N1 infection in children. J Child Neurol 2012; 27:431–8. [DOI] [PubMed] [Google Scholar]
- 17.Lyon JB, Remigio C, Milligan T, Deline C. Acute necrotizing encephalopathy in a child with H1N1 influenza infection. Pediatr Radiol 2010; 40:200–5. [DOI] [PubMed] [Google Scholar]
- 18.Neilson DE, Adams MD, Orr CM, et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet 2009; 84:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin A, Reade EP. Acute necrotizing encephalopathy progressing to brain death in a pediatric patient with novel influenza A (H1N1) infection. Clin Infect Dis 2010; 50:e50–2. [DOI] [PubMed] [Google Scholar]
- 20.Marco EJ, Anderson JE, Neilson DE, Strober JB. Acute necrotizing encephalopathy in 3 brothers. Pediatrics 2010; 125:e693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuguchi M Influenza encephalopathy and related neuropsychiatric syndromes. Influenza Other Respir Viruses 2013; 7(Suppl 3):67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagao T, Morishima T, Kimura H, et al. Prognostic factors in influenza-associated encephalopathy. Pediatr Infect Dis J 2008; 27:384–9. [DOI] [PubMed] [Google Scholar]
- 23.Denier C, Balu L, Husson B, et al. Familial acute necrotizing encephalopathy due to mutation in the RANBP2 gene. J Neurol Sci 2014; 345:236–8. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura N, Higuchi Y, Kimura N, et al. Familial acute necrotizing encephalopathy without RANBP2 mutation: poor outcome. Pediatr Int 2016; 58:1215–8. [DOI] [PubMed] [Google Scholar]
- 25.Singh RR, Sedani S, Lim M, et al. RANBP2 mutation and acute necrotizing encephalopathy: 2 cases and a literature review of the expanding clinico-radiological phenotype. Eur J Paediatr Neurol 2015; 19:106–13. [DOI] [PubMed] [Google Scholar]
- 26.Gika AD, Rich P, Gupta S, et al. Recurrent acute necrotizing encephalopathy following influenza A in a genetically predisposed family. Dev Med Child Neurol 2010; 52:99–102. [DOI] [PubMed] [Google Scholar]
- 27.Grose C The puzzling picture of acute necrotizing encephalopathy after influenza A and B virus infection in young children. Pediatr Infect Dis J 2004; 23:253–4. [DOI] [PubMed] [Google Scholar]