Abstract.
The 15th natural plague focus in China, the Junggar Basin plague focus, is located near an important communication route connecting China and Central Asia and was discovered after 2005. To characterize the phenotypic and genetic diversity of the Yersinia pestis population in this newly established focus, we collected 25 Y. pestis strains from six counties across Junggar Basin in 2005–2006, and determined their biochemical features and genotypes based on multiple-locus variable number of tandem repeats analysis and clustered regularly interspaced short palindromic repeats analysis. We inferred the phylogenetic positions and possible sources of the Junggar strains by comparing their genotypes with the genetic diversity for known representative Y. pestis strains. Our results indicate that the major genotype of Junggar strains belongs to 2.MED1, a lineage of biovar Medievalis with identical biochemical characters and high virulence in mice. Although share a similar ecology, the 2.MED1 in Junggar Basin are not descended from known strains in the neighboring Central Asian Desert plague foci. Therefore, the emergence of the Junggar Basin plague focus is not attributable to the recent clonal spread of Y. pestis from Central Asia. We also identified two distinct minor genotypes in Junggar Basin, one of which clusters genetically with the 0.ANT1 strains of the Tianshan Mountain natural plague focus and another belongs to a 1.IN lineage not previously reported. Our study clarifies the phenotypic and genetic characters of Junggar Y. pestis strains. These findings extend our knowledge of the population diversity of Y. pestis and will facilitate future plague surveillance and prevention in Junggar Basin and adjacent regions.
INTRODUCTION
Natural plague foci, shaped by the interactions among the Yersinia pestis, its hosts, its vectors, and specific environments,1,2 are distributed around the world and generate human plague cases every year through the transmission of the bacterium from infected rodents or fleas.3,4 Yersinia pestis has been active for more than 5,000 years on the Eurasian continent, so natural plague foci may have been established during the Bronze Age.5 During the third pandemic, Y. pestis was spread globally by steam ships, which facilitated the establishment of plague foci in both North and South America, Madagascar, and other regions of Africa.2,6 However, there are few reports of plague foci established after the first half of the 20th century.7,8
Junggar Basin is an enclosed inland basin located between the Altai Mountains and the Tianshan Mountains in northwest China, with an area of approximately 350,000 km.2,9 The second largest desert in China, the Gurbantünggüt Desert, is located at the center of Junggar Basin. A population of great gerbils (Rhombomys opimus) inhabits the whole Desert, extending to the southern edge of the Desert, and feeding on the plant Haloxylon. The commonest flea species on the Junggar great gerbil population are Xenopsylla minax and Xenopsylla skrjabini.9,10 Because its landscape and ecology are very similar to those of the neighboring Central Asian Desert natural plague foci in the former Soviet Union (FSU) region, Junggar Basin has been considered a potential plague focus and was comprehensively investigated in 1956–1977.9 More than 8,000 great gerbils and other rodents and 50,000 parasitic fleas sampled in Junggar Basin were screened for Y. pestis, but no Y. pestis was detected in any of the samples.9,11 In the year 2005, sick and dead great gerbils were found in the Basin during routine surveillance, and Y. pestis strains were isolated from them, suggesting that an animal plague had occurred in this region. A large-scale investigation of the composition of the Y. pestis population in the rodents and their parasitic fleas was undertaken in 2005–2006, in which animal sera were tested for F1 capsule antigen, and Y. pestis bacteria were isolated.10 The results indicated that a new plague focus, with an area of 16,000 km2, had emerged in Junggar Basin, in which the great gerbil was the main host12 and X. skrjabini the main vector.13
Understanding the phenotypic features and genetic diversity of Y. pestis isolates is important in characterizing a plague focus and contributes to the prevention and control of plague. Molecular genotyping and evolutionary analyses were used to describe the phylogenetic positions of the Junggar strains, including a multiple-locus variable number of tandem repeats analysis (MLVA), which is based on the diversity of variable numbers of tandem repeats (VNTR),14,15 typing with clustered regularly interspaced short palindromic repeats (CRISPR), which is based on the varying compositions of the spacer arrays in three CRISPR loci in the genome,16 and whole-genome single-nucleotide polymorphism (SNP) analysis.17 However, only 1–3 Junggar strains were used in those studies, so the fully representative genetic diversity of the Y. pestis population in Junggar Basin focus was not determined.
In this study, we collected 25 Y. pestis strains isolated in Junggar Basin during an investigation in 2005–2006. We determine the biochemical features of these strains, their virulence in animals, and their genetic diversity based on VNTRs and CRISPRs to more comprehensively understand the characteristics of Y. pestis in this newly emerged natural plague focus.
MATERIALS AND METHODS
Bacterial strains and DNA extraction.
Twenty-five Y. pestis strains were isolated from a total of 14,622 biological samples during the investigation10 in Junggar Basin, Xinjiang, in 2005–2006. There are 15 strains were isolated from great gerbils, one from Meriones meridianus, and nine from fleas. The isolation locations included Alashankou (N = 6), Kelamayi (N = 3), Manasi (N = 10), Hutubi (N = 3), Jimusaer (N = 1), and Qitai (N = 2) (Table 1 and Figure 1). All the strains were stored at the Center for Disease Control and Prevention of Xinjiang Uygur Autonomous Region. The strains were cultured in Luria–Bertani broth at 26°C for 48 hours, and the genomic DNAs were extracted with conventional sodium dodecyl sulfate lysis and phenol–chloroform extraction.
Table 1.
Background information and phenotypic features of Yersinia pestis strains in Junggar Basin*
| Strain ID | Source | Location | Isolation time | Tre | Mel | Sal | Ara | Rha | Nr | LD50 |
|---|---|---|---|---|---|---|---|---|---|---|
| XJ2501 | Rhombomys opimus | Hutubi, Xinjiang | 2005 | 1† | 1 | 1 | 1 | 0 | 0 | 13 |
| XJ2502 | Xenopsylla minax | Hutubi, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2503 | Echidnophaga oschanini | Hutubi, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2504 | Rhombomys opimus | Manasi, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2505 | Rhombomys opimus | Manasi, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2507 | Rhombomys opimus | Manasi, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | 93 |
| XJ2508 | Xenopsylla minax | Manasi, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | 18 |
| XJ2510 | Rhombomys opimus | Alashankou, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | 24 |
| XJ2511 | Rhombomys opimus | Alashankou, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | 265 |
| XJ2512 | Xenopsylla minax | Alashankou, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | 30 |
| XJ2513 | Xenopsylla minax | Alashankou, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | > 106 |
| XJ2514 | Xenopsylla minax | Alashankou, Xinjiang | 2005 | 1 | 1 | 1 | 1 | 0 | 0 | > 106 |
| XJ2515 | Rhombomys opimus | Alashankou, Xinjiang | 2005 | 1 | 0 | 1 | 1 | 0 | 0 | < 10 |
| XJ2601 | Xenopsylla minax | Manasi, Xinjiang | 2006 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2602 | Meriones meridianus | Manasi, Xinjiang | 2006 | 1 | 0 | 1 | 1 | 0 | 0 | < 10 |
| XJ2603 | Xenopsylla skrjabini | Manasi, Xinjiang | 2006 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2604 | Xenopsylla conformis | Manasi, Xinjiang | 2006 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2649 | Rhombomys opimus | Qitai, Xinjiang | 2006 | 1 | 0 | 1 | 1 | 0 | 0 | < 10 |
| XJ2650 | Rhombomys opimus | Kelamayi, Xinjiang | 2006 | 1 | 1 | 1 | 1 | 1 | 1 | < 10 |
| XJ2651 | Rhombomys opimus | Manasi, Xinjiang | 2006 | 1 | 0 | 1 | 1 | 0 | 1 | 46 |
| XJ2652 | Rhombomys opimus | Kelamayi, Xinjiang | 2006 | 0 | 1 | 1 | 0 | 1 | 1 | < 10 |
| XJ2653 | Rhombomys opimus | Jimusaer, Xinjiang | 2006 | 1 | 1 | 1 | 1 | 0 | 0 | < 10 |
| XJ2654 | Rhombomys opimus | Manasi, Xinjiang | 2006 | 1 | 0 | 1 | 1 | 0 | 0 | < 10 |
| XJ2655 | Rhombomys opimus | Kelamayi, Xinjiang | 2006 | 1 | 0 | 1 | 0 | 1 | 1 | < 10 |
| XJ2656 | Rhombomys opimus | Qitai, Xinjiang | 2006 | 1 | 0 | 1 | 1 | 0 | 1 | < 10 |
Tre = trehalose; Mel = melibiose; Sal = salicin; Ara = arabinose; Rha = rhamnose; Nr = nitrate reduction.
* Thirteen of the 19 tested biochemical features were completely identical among the 25 strains and are not listed in the table. Fermentation positive: glucose, maltose, mannose, mannitol, glycerol, and fructose. Fermentation negative: erythriol, lactose, saccharose, sorbose, and sorbitol. Also negative for urease activity and VP (Voges–Proskauer).
“1” indicates fermentation positive, and “0” indicates fermentation negative.
Figure 1.
Distribution of Yersinia pestis isolated from the Junggar Basin plague focus. The inset plot indicated the position of Junggar Basin in China. The size of the filled circles or pie chart segments indicates the numbers of Y. pestis strains, the biovars of which are indicated with colors. The rings outside the circles represent the year of isolation (see legend at the top left).
The repeat numbers for 25 VNTR loci in 97 Y. pestis strains representing the genetic diversity of the whole species, four Yersinia pseudotuberculosis strains,15 and 38 Y. pestis strains from FSU plague foci14 were obtained from previously published articles (Supplemental Table 1). The VNTR diversity information for these strains was obtained from the MLVA bank for Microbes Genotyping database (http://microbesgenotyping.i2bc.paris-saclay.fr).18
Phenotyping and virulence testing.
The culture media used to determine the traditional biochemical characteristics were prepared for the sugar fermentation and nitrate reduction experiments. The fermentation tubes were inoculated with Y. pestis strains, which were cultured at 37°C for 3–14 days to observe the biochemical reactions. The median lethal dose (LD50) in BALB/c mice by subcutaneous route was determined for each strain, as previously described.19 The animals were bred in our laboratory, and food and fresh water were given ad libitum. All of the animals were kept in an air-conditioned room with a constant temperature (23 ± 1°C) and humidity (40% ± 10%) under a 12 hours light/dark cycle. All the survival animals were euthanized humanely.
MLVA and CRISPR typing.
The 25 VNTR loci initially reported by Pourcel et al.20 were used in the molecular typing of the Junggar Y. pestis strains. The spacer arrays for all three CRISPR loci in Y. pestis were detected by Sanger sequencing the polymerase chain reaction (PCR) products. The primers and protocols for the PCRs used for both MLVA and CRISPR typing were identical to those reported in previous studies.14–16
Data processing.
Gel images generated with MLVA typing were used as the input for the BioNumerics software (version 6.0; Applied-Maths, Sint-Martens-Latem, Belgium) to calculate the amplicon size, as previously described.14 The results were checked manually. The repeat numbers of the motifs (Supplemental Table 2) were calculated from the corresponding amplicon sizes and information on known alleles. The phylogeny was built with the categorical coefficient and WARD clustering method. Nei’s diversity index (DI) for each VNTR locus was calculated with the equation: DI = 1 − ∑ (allele frequency).2
The spacers at the CRISPR loci were recognized by comparing them with a spacer dictionary, as in a previous study,16 and the CRISPR genotypes were defined according to the spacer array compositions of all three CRISPR loci (Supplemental Table 3).
Ethics statement.
This study was approved by the ethics committee of the Center for Disease Control and Prevention of the Xinjiang Uygur Autonomous Region in China (Permit Number: DWLL-XJ-07012). All animal experiments were carried out in strictly accordance with the recommendations in the Regulations of Good Laboratory Practice for nonclinical laboratory studies of drug issued by the National Scientific and Technologic Committee of People’s Republic of China.
RESULTS
Phenotypic features.
To characterize the phenotypes of the Junggar isolates, we determined their virulence in mice, their nitrate reduction capacities, and their fermentation of 18 types of nutrients, including glycerol, rhamnose, arabinose, etc. Most strains were highly virulent in mice, and more than half the strains displayed LD50 < 10 colony-forming units (CFU). Excluding two outlier strains, the largest LD50 for the strains was 265 CFU (Table 1). According to the traditional biovar definitions for Y. pestis,21 20 of the 25 strains were biovar Medievalis because they were positive for glycerol fermentation and negative for nitrate reduction. The other five strains were biovar Antiqua because they were positive for both glycerol fermentation and nitrate reduction. The Medievalis strains displayed a homogeneous phenotype. Only three of the 20 strains were melibiose negative, whereas the fermentation profiles of the other strains were completely consistent. Although only five strains were assigned to biovar Antiqua, they showed different fermentation capacities on trehalose, melibiose, arabinose, and rhamnose, indicating that the Antiqua strains in Junggar Basin are more phenotypically polymorphic than the Medievalis strains.
Molecular genotyping.
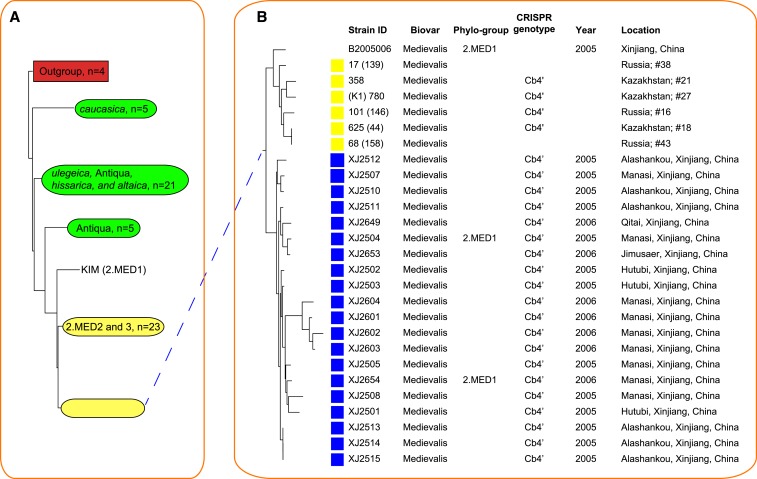
To define the phylogenetic positions of the Junggar strains in the Y. pestis genealogy, we used the MLVA method, which is based on the polymorphisms of 25 VNTR loci, to construct a neighbor-joining (NJ) tree for the 25 Junggar strains, together with a set of 97 strains that represented most of the genetic diversity in Y. pestis species.15 Four Y. pseudotuberculosis strains were used as the outgroup (Figure 2A and B and Supplemental Figure S1). In the phylogeny, the majority of Junggar strains (20 of 25, 80%), all belonging to biovar Medievalis, clustered with 2.MED1 and were therefore assigned to this phylogroup. By screening the nucleotide sequence of napA613, a signature mutation of 2.MED strains,22,23 we found that the premature stop codon of napA was present in all 20 Junggar Medievalis strains, confirming the group definition. The whole genomic sequences of two Junggar strains, XJ2504 and XJ2654, have been previously determined with the next-generation sequencing technology, and a phylogenomic analysis also assigned these Junggar strains to the 2.MED1 lineage.17
Figure 2.
Neighbor-joining (NJ) tree of 25 Junggar strains and 97 strains representing the whole species diversity of Yersinia pestis. The tree was constructed based on the diversity of 25 variable numbers of tandem repeats loci, and four Yersinia pseudotuberculosis strains were used as the out group. (A) NJ dendrogram of 126 strains, with collapsed branches for visual effect. The complete tree is shown in Supplemental Figure 1. (B) The branch that includes the major genotype of the Junggar strains (represented by blue squares before the strain ID) and its neighboring lineage is shown. Strains indicated with red font have been sequenced previously and their draft genomes are available. (C) The branch that includes the minor genotypes (represented by red squares before the strain ID) of the Junggar strains and their neighboring lineages is shown. CRISPR = clustered regularly interspaced short palindromic repeats.
The five Junggar Antiqua strains were distributed in two distinct lineages (Figure 2C), suggesting greater genetic diversity than in the Junggar Medievalis strains, which is consistent with their greater phenotypic polymorphism. Strains XJ2652 and XJ2655 belong to the 0.ANT1 lineage, which contains a group of strains predominantly isolated at the Tianshan Mountain natural plague focus, also known as Focus B, Xinjiang (Supplemental Table 3).14,24,25 The region-specific spacer, Ca37, of Focus B was identified at the CRISPR YPa locus in both strains, providing further evidence that these two strains have similar genetic backgrounds to those of the Antiqua strains previously isolated in the Tianshan Mountains.16
Interestingly, the three other Junggar Antiqua strains, XJ2650, XJ2651, and XJ2656, which occur midway between the 1.IN and 1.ORI groups on the NJ tree (Figure 2C), form a lineage that was observed here for the first time in Y. pestis. This new lineage is the same biovar and CRISPR genotype (Ca7) as the strains in the 1.IN group, suggesting that it is more closely related to the 1.IN group than to the 1.ORI group. Therefore, we designated it 1.IN4.
Junggar strains did not spread clonally from FSU plague foci.
It has been supposed that the Medievalis strains in Junggar Basin were transmitted from the natural Central Asian Desert plague foci of the FSU because the two regions share highly similar ecologies and therefore provide identical niches for specific subgroups of Y. pestis. To test this hypothesis, we collected previously published VNTR information for 38 strains isolated from the FSU14 and 28 Medievalis strains with known phylogroup designations,15 and used them, together with our 20 Junggar Medievalis strains, to construct an NJ tree (Figure 3 and Supplemental Figure 2). The phylogeny indicated that the Junggar strains constitute a sister lineage to a group of FSU strains that were predominantly isolated from the natural plague foci in the northern Pre-Caspian region (Foci 16 and 43) and the Central Asian Desert region (Foci 18, 21, and 27), in which gerbil species (M. meridianus and R. opimus, respectively) are the main host species.26 The CRISPR genotypes of the sister lineages are both Cb4’, further confirming the close relationship between the two groups of strains. However, the Junggar strains are not the direct descendants of FSU strains, and the genetic diversity (measured with Nei’s diversity index of the VNTRs) of the FSU strains is not higher than that of the Junggar strains (Mann–Whitney U test, P = 0.28; Supplemental Figure 3). Therefore, it is impossible that the Junggar strains represent a clone that recently spread from the natural plague foci in the FSU.
Figure 3.
Neighbor-joining (NJ) tree of the major genotypes of the Junggar strains, representative Medievalis strains, and former Soviet Union (FSU) strains of Yersinia pestis. Because data were missing for two variable numbers of tandem repeats (VNTR) loci (YPO1108ms45 and YPO1118ms69) in six FSU strains, the NJ tree was constructed based on the remaining 23 VNTR loci. (A) NJ dendrogram of 86 strains, with collapsed branches for visual effect. The phylogroup designations of most FSU isolates are unknown; hence, only their biovars are indicated on the collapsed branches and colored by green shade. The biovar designation of strains of caucasica, altaica, hissarica, and ulegeica are acquired from reference 38. The complete tree is shown in Supplemental Figure 2. (B) The branch that includes the Junggar strains and its sister lineage is shown. Blue squares before the strain IDs indicate Junggar strains and yellow squares indicate FSU strains. CRISPR = clustered regularly interspaced short palindromic repeats.
DISCUSSION
In this study, we determined the phenotypic characters and genotypes of Y. pestis strains isolated from the newly emergent plague focus in Junggar Basin. The major genotype of the Junggar strains belonged to the 2.MED1 group, which is characterized by glycerol fermentation without nitrate reduction, and is relatively highly virulent in mice. The strains distributed across the whole Junggar Basin were isolated in two consecutive years, suggesting that this group of strains has established stable cycles on local hosts and vectors and is therefore shaping the natural plague focus. This was confirmed by subsequent surveillance, with the punctuated isolation of Y. pestis strains from the great gerbil and its parasitic fleas in the region.27,28
Because no Y. pestis was isolated during the comprehensive investigation of Junggar Basin during the 1950s–1970s, it is reasonable to infer that the establishment of the new focus was caused by the input of Y. pestis strains from adjoining natural plague foci, which occupy niches identical to that of the new focus. However, our results suggest that the Junggar strains are not the direct clonal descendants of the known Y. pestis strains in the plague foci of the Central Asian Desert. The other known Central Asian isolate from the 2.MED1 group, KIM,29 also belongs to a lineage distinct from the Junggar strains, differing by 28 SNPs.17 Therefore, it seems that the emergent Junggar Basin plague focus is not a progeny focus of the Central Asian plague foci, arising from a recent transmission event, as previously assumed. We propose two possible scenarios for the emergence of this new plague focus. In the first, the plague focus has always been present in Junggar Basin, but experienced a long enzootic period that lasted dozens of years, in which negligible Y. pestis was found in either hosts or fleas.30,31 The focus has now entered an epizootic phase, leading to the frequent isolation of Y. pestis in the region. In the alternative scenario, Y. pestis derives from an unknown source, such as Focus 29 or Focus 30 in Kazakhstan, with great gerbils as the main host and Xenopsylla gerbilli as the vector.26 However, no genetic information on isolates from these foci is yet available. Therefore, more samples from the surrounding regions are required to determine their genetic diversity if we are to trace the source of the emergent or reemergent Junggar Basin plague focuses.
Besides the major genotype, 2.MED1, there are two minor genotypes of the Junggar strains were also observed in this study. One of the minor genotypes included two biovar Antiqua strains that were isolated from great gerbils in the city of Kelamayi, and clustered with the Y. pestis 0.ANT1 strains, which are mainly found at the Tianshan Mountain plague focus (Supplemental Table 4). The Tianshan plague focus is located approximately 100 km south of Kalamayi, with the main hosts in the Tianshan Mountain plague focus are Citellus undulatus and Marmota baibacina, and the main vector is Oropsylla silantiewi.11,32 The long-distance spread of Y. pestis strains via the natural migrations of their hosts and/or vectors is usually difficult; however, their spread by other factors is more likely, such as the seasonal movements of nomads and their livestock between pastures. Alternative explanation for the transmission of Y. pestis strains is ecological changes occurred during recent years. As described in previous works, the climate changes are associated with population fluctuation of hosts and vectors33 and possibly accelerated transmission of human plague34 and caused long-distance spread of Y. pestis.35 The environmental information, including temperature, rainfall, etc., during recent years in the region should be collected to verify the hypothesis of ecological-driven spread of Junggar strains in future studies.
The other minor genotype of the Junggar strains identified in this study belongs to the first-observed Y. pestis lineage, here designated 1.IN4, which emerged before the appearance of the 1.ORI group of strains. According to previous research, the 1.IN group strains mainly survived on the Qinghai–Tibet Plateau. A subgroup of the 1.IN strains spread south to Yunnan Province and evolved into the 1.ORI group there.6,25 This southward transmission later spread the bacterium even further to Hong Kong,36 ultimately disseminating globally to cause the third plague pandemic.1,6,26 The southern transmission of the 1.IN strains is supported by the identification of strains closely related to 1.IN in YuLong county, Yunnan Province, based on CRISPR and pulsed-field gel electrophoresis analyses.37 Here, the presence of the 1.IN4 lineage indicates that 1.IN strains not only moved south, but also moved to the northwest of China, arriving in Xinjiang, where they survive to this day. This suggests that multiple waves of plague originated on the Qinghai–Tibet Plateau throughout history. The whole-genome sequences of the 1.IN4 strains will be required to decipher the evolutionary details of this newly identified Y. pestis lineage.
CONCLUSIONS
In this study, we determined the phenotypic and genetic diversity of Y. pestis strains isolated in Junggar Basin in 2005–2006. Our results show that 2.MED1, the major genotype of the newly emergent Junggar Basin plague focus, is similar to but did not recently originate from the Y. pestis strains of the Central Asian Desert plague foci. Three independent lineages, included a new lineage of Y. pestis, 1.IN4, were observed in Junggar Basin, suggesting enhanced surveillance of Y. pestis is required to improve the prevention and control of plague in this region. More strain samples, combined with a broad whole-genome analysis of genetic diversity, are required to infer the accurate sources and evolutionary scenarios of both the 2.MED1 and 1.IN4 strains present in the Junggar Basin plague focus.
Supplementary Material
Supplemental Table and Figure.
Note: Supplemental figures and tables appear at www.ajtmh.org.
REFERENCES
- 1.Perry RD, Fetherston JD, 1997. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev 10: 35–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, Carniel E, Gage KL, Leirs H, Rahalison L, 2008. Plague: past, present, and future. PLoS Med 5: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinnebusch BJ, 2005. The evolution of flea-borne transmission in Yersinia pestis. Curr Issues Mol Biol 7: 197–212. [PubMed] [Google Scholar]
- 4.Lorange EA, Race BL, Sebbane F, Joseph Hinnebusch B, 2005. Poor vector competence of fleas and the evolution of hypervirulence in Yersinia pestis. J Infect Dis 191: 1907–1912. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen S, et al. 2015. Early divergent strains of Yersinia pestis in Eurasia 5,000 years ago. Cell 163: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morelli G, et al. 2010. Yersinia pestis genome sequencing identifies patterns of global phylogenetic diversity. Nat Genet 42: 1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Zhang J, Chen F, 1981. The Epidemic history of Plague, China. Beijing, China: Institute of Epidemiology and Microbiology, Chinese Academy of Medical Science. [Google Scholar]
- 8.WHO , 2000. WHO Report on Global Surveillance of Epidemic-Prone Infectious Disease Chapter 3. Geneva, Switzerland: World Health Organization, 25–37. [Google Scholar]
- 9.Xin Y, 2007. Outline of investigation on plague natural focus of Rhombomys opimus in Junggar Basin, Xinjiang. Endemic Dis Bull 22: 57–60. [Google Scholar]
- 10.Zhang YJ, et al. 2008. Study on the situation of plague in Junggar Basin of China. Zhonghua Liu Xing Bing Xue Za Zhi 29: 136–144. [PubMed] [Google Scholar]
- 11.Ji S, 1988. Plague. Beijing, China: People’s medical publishing house. [Google Scholar]
- 12.Zhang Y, et al. 2012. Dynamics of Yersinia pestis and its antibody response in great gerbils (Rhombomys opimus) by subcutaneous infection. PLoS One 7: e46820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. 2015. Transmission efficiency of the plague pathogen (Y. pestis) by the flea, Xenopsylla skrjabini, to mice and great gerbils. Parasit Vectors 8: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, et al. 2009. Genotyping and phylogenetic analysis of Yersinia pestis by MLVA: insights into the worldwide expansion of central Asia plague foci. PLoS One 4: e6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, et al. 2013. Features of variable number of tandem repeats in Yersinia pestis and the development of a hierarchical genotyping scheme. PLoS One 8: e66567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui Y, et al. 2008. Insight into microevolution of Yersinia pestis by clustered regularly interspaced short palindromic repeats. PLoS One 3: e2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y, et al. 2013. Historical variations in mutation rate in an epidemic pathogen, Yersinia pestis. Proc Natl Acad Sci USA 110: 577–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grissa I, Bouchon P, Pourcel C, Vergnaud G, 2008. On-line resources for bacterial micro-evolution studies using MLVA or CRISPR typing. Biochimie 90: 660–668. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Zhang Y, Hu Y, 2008. Laboratory Animal Technology in Medicine. Shaanxi, China: The Fourth Military Medical University Publishing House. [Google Scholar]
- 20.Pourcel C, Andre-Mazeaud F, Neubauer H, Ramisse F, Vergnaud G, 2004. Tandem repeats analysis for the high resolution phylogenetic analysis of Yersinia pestis. BMC Microbiol 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Devignat R, 1951. Varieties of Pasteurella pestis; new hypothesis. Bull World Health Organ 4: 247–263. [PMC free article] [PubMed] [Google Scholar]
- 22.Achtman M, et al. 2004. Microevolution and history of the plague bacillus, Yersinia pestis. Proc Natl Acad Sci USA 101: 17837–17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou D, et al. 2004. Genetics of metabolic variations between Yersinia pestis biovars and the proposal of a new biovar, microtus. J Bacteriol 186: 5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou D, et al. 2004. DNA microarray analysis of genome dynamics in Yersinia pestis: insights into bacterial genome microevolution and niche adaptation. J Bacteriol 186: 5138–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, et al. 2008. Different region analysis for genotyping Yersinia pestis isolates from China. PLoS One 3: e2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anisimov AP, Lindler LE, Pier GB, 2004. Intraspecific diversity of Yersinia pestis. Clin Microbiol Rev 17: 434–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao H, Zhang Y, liu Q, 2012. Control surveillance and epidemic analysis of plague in Xinjiang, 2011. Bulletin of Disease Control and Prevention 27: 28–33. [Google Scholar]
- 28.Guo R, et al. 2014. Study on the spatial and temporal distribution of animal plague in Junggar Basin plague focus. Zhonghua Liu Xing Bing Xue Za Zhi 35: 109–113. [PubMed] [Google Scholar]
- 29.Deng W, et al. 2002. Genome sequence of Yersinia pestis KIM. J Bacteriol 184: 4601–4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gage KL, Kosoy MY, 2005. Natural history of plague: perspectives from more than a century of research. Annu Rev Entomol 50: 505–528. [DOI] [PubMed] [Google Scholar]
- 31.Drancourt M, Houhamdi L, Raoult D, 2006. Yersinia pestis as a telluric, human ectoparasite-borne organism. Lancet Infect Dis 6: 234–241. [DOI] [PubMed] [Google Scholar]
- 32.Fang XY, et al. 2012. Ecological-geographic landscapes of natural plague foci in China VII. Typing of natural plague foci. Zhonghua Liu Xing Bing Xue Za Zhi 33: 1144–1150. [PubMed] [Google Scholar]
- 33.Stenseth NC, et al. 2006. Plague dynamics are driven by climate variation. Proc Natl Acad Sci USA 103: 13110–13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L, Stige LC, Kausrud KL, Ben Ari T, Wang S, Fang X, Schmid BV, Liu Q, Stenseth NC, Zhang Z, 2014. Wet climate and transportation routes accelerate spread of human plague. Proc Biol Sci 281: 20133159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid BV, Buntgen U, Easterday WR, Ginzler C, Walloe L, Bramanti B, Stenseth NC, 2015. Climate-driven introduction of the Black Death and successive plague reintroductions into Europe. Proc Natl Acad Sci USA 112: 3020–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yersin A, 1894. La peste bubonique à Hong-Kong. Ann Inst Pasteur (Paris) 2: 428–430. [Google Scholar]
- 37.Wang P, Li W, Zhang Z, Guo Y, Shi L, Ye R, Cui Z, Yang G, Dong S, Song Z, 2016. Characters of Yulong Yersinia pestis strains from Yunnan province, China. Int J Clin Exp Med 9: 6394–6402. [Google Scholar]
- 38.Platonov ME, et al. 2015. Intraspecies classification of rhamnose-positive Yersinia pestis strains from natural plague foci of Mongolia. Mol Gen Microbiol Virol 30: 24–29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table and Figure.