Abstract
From 2006 to 2013, an increasing incidence of fusariosis was observed in the hematologic patients of our University Hospital. We suspected of an environmental source, and the indoor hospital air was investigated as a potential source of the fungemia. Air samplings were performed in the hematology and bone marrow transplant (BMT) wards using an air sampler with pre-defined air volumes. To study the molecular relationship among environmental and clinical isolates, 18 Fusarium spp. recovered from blood cultures were included in the study. DNA sequencing of a partial portion of TEF1α gene was performed for molecular identification. Molecular typing was carried out by multi-locus sequence typing (MLST) using a four-gene scheme: TEF1α, rDNA, RPB1 and RPB2. One hundred four isolates were recovered from the air of the hematology (n = 76) and the BMT (n = 28) wards. Fusarium isolates from the air were from five species complexes: Fusarium fujikuroi (FFSC, n = 56), Fusarium incarnatum-equiseti (FIESC, n = 24), Fusarium solani (FSSC, n = 13), Fusarium chlamydosporum (FCSC, n = 10), and Fusarium oxysporum (FOSC, n = 1). Fifteen Fusarium isolates recovered from blood belonged to FSSC, and three to FFSC. MLST identified the same sequence type (ST) in clinical and environmental isolates. ST1 was found in 5 isolates from blood and in 7 from the air, both identified as FSSC (Fusarium petroliphilum). STn1 was found in one isolate from blood and in one from the air, both identified as FFSC (Fusarium napiforme). F. napiforme was isolated from the air of the hospital room of the patient with fungemia due to F. napiforme. These findings suggested a possible clonal origin of the Fusarium spp. recovered from air and bloodcultures. In conclusion, our study found a diversity of Fusarium species in the air of our hospital, and a possible role of the air as source of systemic fusariosis in our immunocompromised patients.
Introduction
Filamentous fungi of the genera Fusarium are ubiquitous in the environment and are found in the soil, water, and air [1]. Fusarium species are primarily plant pathogens, and produce toxins that can cause food poisoning[2]. Fusarium can also infect humans and cause localized or disseminated diseases depending on the predisposing factors and the immunological status of the host [3, 4]. Keratitis[2] and onychomycosis[5] are the most common infections in the immunocompetent hosts, and the infections may also occur because of trauma or by the use of contaminated contact lens[6, 7].
In the immunocompromised patients, especially those with hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation (HSCT), Fusarium can disseminate in the organism causing a systemic and invasive infection (fusariosis) [3, 8, 9]. Fusarium solani is the most frequent species involved in fusariosis (50% of cases), followed by Fusarium oxysporum (20%) and Fusarium verticillioidis and Fusarium moniliforme (10% each). In Brazil, Fusarium is the leading cause of invasive mold infections, followed by Aspergillus, with an overall incidence of 6 cases per 1,000 HSCTs[3, 10, 11]. Fusarium spp. may show an antifungal susceptibility profile marked by high level of resistance; however some isolates can be susceptible in vitro to amphotericin B, voriconazol[12, 13], notwithstanding, the mortality rate iof disseminated fusariosis may exceed 75%.
For severely immunocompromised patients, hospitalization in a controlled environment has been recommended, such as in private rooms equipped with high-efficiency particulate air (HEPA) filters and positive airflow system. Several studies evaluated the effectiveness of HEPA filters in preventing or reducing invasive aspergillosis in hematologic and oncologic patients[14, 15], but did not evaluate other filamentous fungi, such as Fusarium.
From 2006 to 2013, 34 patients from the hematology and the bone marrow transplant (BMT) wards were diagnosed with invasive fusariosis in our University Hospital, with an incidence density during this period ranging from 0.18 to 2.4 per 1,000 patients-day (incidence density in 8 years: 0.82 cases per 1,000 patients-day). Fusariosis, in our hospital, was surprisingly higher than most hospitals worldwide[11] raising the hypothesis that an environmental source could be implicated in the high incidence of fusariosis. Therefore, we studied the air as a potential environmental source for invasive fusariosis by comparing the genetic relationship of Fusarium isolates obtained from the environment, as well as the ones obtained from blood cultures.
Materials and methods
Study location
This study was performed at the Clinical Hospital of the University of Campinas, Campinas, Sao Paulo, Brazil. It is a 419-bed tertiary-care university hospital and is the referral hospital for all major medical services in an area of 3,000,000 inhabitants. The BMT ward has 7 rooms with 9 beds, HEPA filters and positive pressure airflow. The hematology ward has 9 rooms with 11 beds and no controlled air. All patients with hematologic malignancies were hospitalized in this ward and patients that underwent HSCT were hospitalized in the BMT. As we had an increasing number of systemic fusariosis in patients with hematologic malignancies, we assumed that we were having an outbreak with a potential source that needed a prompt investigation by the Infection Control Team and the Mycology Laboratory. The Infection Control Division performed the air samplings without consulting the University Ethical Committee, as that investigation of potential sources of outbreaks is mandatory.
Clinical isolates
Eighteen isolates of Fusarium obtained from 15 patients with hematologic diseases, between 2007 and 2013, were included in this study. Fusarium species were isolated from blood cultures by using the Bact/ALERT (BioMérieux, France) and subsequent morphology evaluation in Sabouraud Dextrose Agar medium (Difco, USA)[16]. Fifteen isolates were from individual patients and three isolates were from three different blood samples withdrawn from the same patient. All samples were collected for routine diagnostic exams and no clinical information was collected from the patients’ records. The clinical isolates numbers: 917, 952, 1192, 1549, 1601, 1603, 1631, 1750, 2020, 916, 1103,1202, 1207, 1372 and 1554 were isolated from patients hospitalized from July 2007 to July 2011, and they were stored in the Mycology Laboratory Culture Collection. As these isolates belonged to a Culture Collection, there was no need for ethical approval. The isolates number 2008, 2009 and 2010 were from patients that participated in the Project CAAE 0870.0.146.000–11 approved by the Ethical Committee Decision No. 964/2011 (Principal investigators: M. de Sousa and P. Trabasso)[17].
Air sampling
The air samplings were performed from March 2012 to March 2013 at the hematology and BMT wards. The samplings were performed during summer, autumn, and spring seasons. The air was collected by the air sampler Bio Samp Model MBS 1000D (Yotsubishi Corp., Japan) in a selective culture medium for Fusarium modified by Mikami Y., Chiba University, Japan[18]. The volume of 1,000 L and 500 L of air was collected from the BMT and hematology wards, respectively. All the samples were taken approximately 1.5 m above the floor and the air was sampled three times in the same room and once in the bathroom. Air isolates were inoculated in Sabouraud Dextrose Agar medium (Difco, USA). Temperature and humidity during sampling were also recorded. The plates were incubated at 37°C for 15 days and fungi with micro and macro morphology resembling Fusarium spp. and the strains for working stock were stored in distilled water[16, 19].
Molecular identification
DNA extraction
Strains were transferred to Sabouraud Dextrose Agar medium (Difco, USA) and incubated at room temperature for 7 days. DNA was extracted using the QiaAmp DNA Mini Kit (Qiagen, USA), according to the manufacturer’s instructions.
PCR reactions
PCR was performed using specific primers in order to amplify four genes fragments chosen for Fusarium identification and MLST: TEF1α (translation elongation factor—1α), rDNA (ribosomal DNA), RPB1 (RNA polymerase largest subunit) and RPB2 (RNA polymerase second largest subunit). The position and sequences of the primers used in this study are described in Table 1. PCR was performed using PCR Master Mix (Promega, USA). PCR reactions were incubated in a Veriti 96 well Thermal Cycler (Applied Biosystem, USA) under the following conditions: 2 min of initial denaturation at 98°C, 40 cycles of DNA denaturation at 98°C for 30 s, primer annealing temperature varying according to the target gene for 30 s, elongation at 72°C for 1 min and a final elongation step at 72°C for 5 min. PCR products were verified by electrophoresis in a 2% agarose gel, 100 v for 30 min. PCR products were purified with ExoSAP-IT for PCR Product Clean-up (Affymetrix USB, USA) prior to sequencing analysis.
Table 1. Primers used for sequencing of clinical and environmental Fusarium isolates.
| Gene | Protein | Primer | Reference | |
|---|---|---|---|---|
| Name | Sequence (5' - 3')a | |||
| TEF1α | Translation elongation factor 1 alpha | HS392 | TCAAAATGGGTAAGGA(A/G)GACAAGAC | [20, 21] |
| HS393 | GCCTGGGA(A/G)GTACCAGT(C/G)ATCATGTT | [20, 21] | ||
| EF11 | GTGGGGCATTTACCCCGCC | [21] | ||
| EF21 | GAGTGGCGGGGTAAATGCC | [21] | ||
| rDNA | Ribosomal DNA | ITS4 | TCCTCCGCTTATTGATATGC | [23] |
| ITS5 | GGAAGTAAAAGTCGTAACAAGG | [24] | ||
| NL1 | GCATATCAATAAGCGGAGGAAAAG | [23] | ||
| NL4 | GGTCCGTGTTTCAAGACGG | [24] | ||
| RPB1 | RNA polymerase largest subunit | Fa | CAYAARGARTCYATGATGGGWC | [25] |
| F5 | ATGGGTATYGTCCAGGAYTC | [25] | ||
| F7 | CRACACAGAAGAGTTTGAAGG | [25] | ||
| F8 | TTCTTCCACGCCATGGCTGGTCG | [25] | ||
| R8 | CAATGAGACCTTCTCGACCAGC | [25] | ||
| G2R | GTCATYTGDGTDGCDGGYTCDCC | [25] | ||
| R9 | TCARGCCCATGCGAGAGTTGTC | [25] | ||
| F2 | GATGGGATCGBGCHTTYGTCA | This study | ||
| F1c | GACTGGTTCAAGCATGACTACGAAT | This study | ||
| F1e | CGACAAGTGCGACAGATTAACAAGG | This study | ||
| RPB2 | RNA polymerase second largest subunit | 6F | TGGGGKWTGGTYTGYCCTGC | [26] |
| 5F2 | GGGGWGAYCAGAAGAAGGC | [24] | ||
| 7cR | CCCATRGCTTGYTTRCCCAT | [24] | ||
| 7cF | ATGGGYAARCAAGCYATGGG | [24] | ||
| 11aR | GCRTGGATCTTRTCRTCSACCC | [24] | ||
| 40R | AGCTTGCGTCCAGTATGACC | [23] | ||
| 40-2F | CAAAAACCTCTGGCGACAAC | [23] | ||
| 2F | ATTTGCATGACKCCNGARGATC | This study | ||
| 2R | ACTRCTCTGGTTCATAATGACGGAA | This study | ||
bp: base pairs.
a Y: C or T; W: A or T; R: A or G; K: G or T; S: G or C; D: A, G or T; B: G, T or C; H: A, C or T.
DNA sequencing for identification
A partial portion of TEF1α was sequenced with the BigDye Terminator reagent kit (Applied Biosystems, USA) in an ABI Prism 3,100 Genetic Analyzer (Applied Biosystems, USA) using HS392, HS393, EF11 and EF21 primers [20, 21] (Table 1). DNA sequences were edited and assembled by Sequencher version 5.2.4 (Gene Codes, USA). For identification, a homology search for the sequences of TEF1α gene was done using the BLAST tool of the NCBI database (GenBank), the database FUSARIUM-ID (http://isolate.fusariumdb.orgl/), and the Fusarium CBS database (http://www.cbs.knaw.nl/fusarium). To confirm the identity of our Fusarium species, we evaluated their position with maximum likelihood (ML) method and a tree of TEF1α analysis was constructed. In these analyses, our sequences, together with sequences retrieved from GenBank and CBS database, were analyzed. Consensus sequences were computed with SeqMan from the Lasergene package (DNA Star, USA). Sequences were aligned with the program MAFFT (www.ebi.ac.uk/Tools/msa/mafft/), followed by manual adjustments with MEGA 6 [22] and BioEdit v7.0.5.2.
Multi Locus Sequencing Typing (MLST) for isotyping
Fusarium isolates found in air and blood from to the same species or species complex were submitted to MLST. Portions of the following four genes fragments were chosen for MLST: TEF1α (598 bp), rDNA (1,029 bp), RPB1 (2,705 bp) and RPB2 (1,750 bp) (Table 1; S1 Fig). The number of loci used for MLST was based on the previous studies of Scheel et al [23]which include TEF1α, rRNA and RPB2 and O’Donnell et al [27] that used TEF1α, RPB1 and RPB2 for phylogenetic analysis. We combined the loci described by both authors to generate a 4-loci scheme with more robust genetic typing. These four portions were sequenced in an ABI Prism 3,100 Genetic Analyzer (Applied Biosystems, USA) using primers described before[20, 21] and additional designed primers (Table 1). DNA sequences were edited and assembled by Sequencher version 5.2.4 (Gene Codes, USA). Sequences for all genes (6,082bp) were aligned by Clustal W tool and followed manual adjustments with MEGA6[22]. Numbers were assigned to each allelic variant and combined in order to generate a unique sequence type (ST) for every Fusarium isolate. The sequence data obtained in this study was deposited in GenBank and the accession numbers are listed in S1 Table.
Results
Air sampling and climatic conditions
We performed nine air samplings from 2012 to 2013. Five air samplings were positives for the recovery of Fusarium spp. and four samplings resulted negatives (S2 Table). One hundred and four isolates were recovered from hematology (n = 76; 73.1%) and BMT units (n = 28; 26.9%). The median temperature during the sampling days was 30.4 ± 3.68 (°C) and the median humidity varied from 49.0 ± 17.2 to 72.0 ± 15.4 (oC). No relationship was found between the dates of air samplings, climate conditions, and season of the year, and the number of Fusarium species isolated from the hospital air.
Identification by TEF1α sequencing
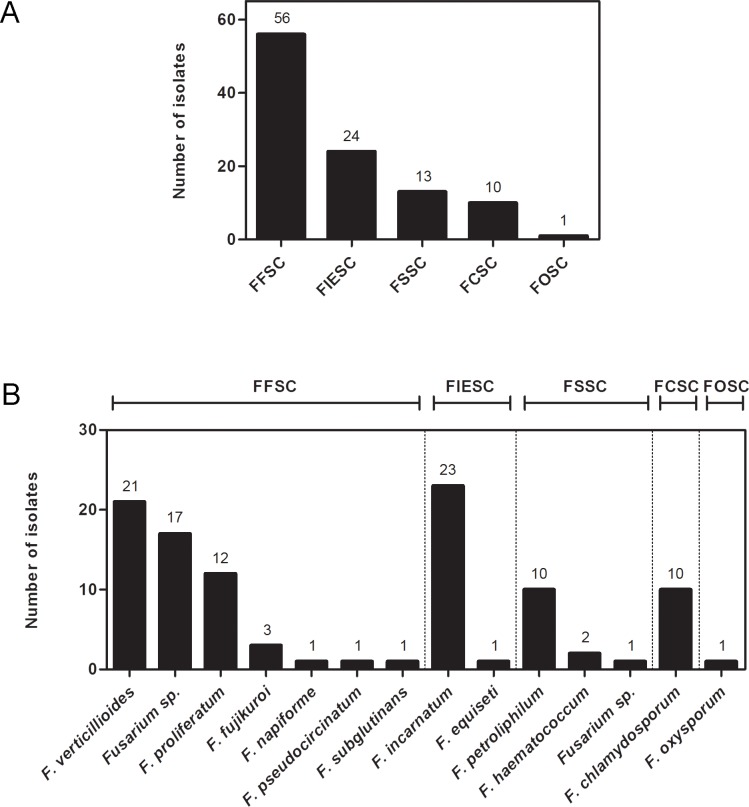
The DNA sequencing of a portion of TEF1α gene was performed for air (n = 104) and clinical (n = 18) Fusarium isolates. Results of phylogenetic analysis of the 104 strains from air assigned 86 strains to species level, belonging to five species complexes: F. solani (FSSC), Fusarium fujikuroi (FFSC), Fusarium oxysporum (FOSC), Fusarium incarnatum-equiseti (FIESC) and Fusarium chlamydosporum (FCSC) (Fig 1, S2–S4 Figs). The most common species of FFSC isolated from air was Fusarium verticillioides (n = 21 isolates), followed by Fusarium proliferatum (n = 12), F. fujikuroi (n = 3), Fusarium napiforme (n = 1), Fusarium pseudocircinatum (n = 1), and Fusarium subglutinans (n = 1). The species of FSSC isolated from air were Fusarium petroliphilum (n = 10) and Fusarium haematococcum (n = 2). We also recovered 24 isolates of FIESC (23 Fusarium incarnatum and 1 Fusarium equiseti), 10 FCSC (F. chlamydosporum), and 1 FOSC (F. oxysporum). Eighteen isolates from the air were not assigned to species level: FFSC (n = 17), and FSSC (n = 1), and formed well-supported monophyletic branches suggesting that further phylogenetic work is necessary for species delimitation and description for these isolates (Fig 1, S2–S4 Figs).
Fig 1. Molecular identification of Fusarium species isolated from hospital air samplings.
(A) and (B) shows TEF1α DNA sequencing classification in species complex and species, respectively. The number of isolates is shown above each bar. FCSC: F. chlamydosporum species complex; FFSC: F. fujikuroi species complex; FIESC: F. incarnatum-equiseti species complex; FOSC: F. oxysporum species complex; FSSC: F. solani species complex.
Our results also showed that air isolates that belong to FFSC were predominant in the hematology unit (n = 46 isolates, 60.5%), followed by FIESC (n = 21, 27.6%), FSSC (n = 4, 5.3%), FCSC (n = 4, 5.3%), and FOSC (n = 1, 1.3%) (Fig 2). In the BMT, FFSC isolates were predominant (n = 10, 35.7%), followed by FSSC (n = 9, 32.1%), FCSC (n = 6, 21.4%), and FIESC (n = 3, 10.7%).
Fig 2. Distribution of Fusarium species isolated from hospital air.
The frequency of each species complex in the hematology (A, n = 76) and BMT (B, n = 28) wards is shown. The species identified for each complex is presented outside the graphs. The species found exclusively in hematology unit are marked with (*). FCSC: F. chlamydosporum species complex; FFSC: F. fujikuroi species complex; FIESC: F. incarnatum-equiseti species complex; FOSC: F. oxysporum species complex; FSSC: F. solani species complex.
Among 18 Fusarium isolates from blood cultures, 15 belonged to FSSC and three isolates to FFSC. The phylogenetic analysis classified the clinical isolates into F. petroliphilum (FSSC, n = 9 isolates), F. keratoplaticum (FSSC, n = 5), and F. napiforme (FFSC, n = 3) (S2 and S3 Figs). The phylogenetic tree also presented the monophyly of one undefined species of FSSC (isolate 1554) (S3 Fig). Detailed information about the Fusarium isolates (isolation date, ward, room, and sequencing identification) is shown in S1 Table.
Molecular typing of clinical and air isolates
Fusarium isolates belonging to FSSC and F. napiforme (FFSC) recovered from blood (16 isolates) and from the air (12 isolates) were submitted to clonal origin analysis (MLST). Thirteen distinctive STs were determined based on the four-locus dataset (6,082 bp): 2 STs for F. napiforme isolates (STn1 and STn2) and 11STs for FSSC (ST1 to ST11) (Fig 3). Eight STs were assigned only for blood Fusarium isolates (ST4 to ST9, ST11 and STn2), and 3 STs were found exclusively in isolates from the air (ST2, ST3 and ST10).
Fig 3. Sequence types (ST) determined by sequencing of portions of the genes TEF1α, rDNA, RPB1 and RPB2 for Fusarium species isolated from air and blood.
The number of samples with each ST is shown above the bar. FSSC: F. solani species complex. FFSC: F. fujikuroi species complex. ST: sequence type.
ST1 and STn1 were found in clinical and environmental isolates and determined a possible clonal origin between blood and air Fusarium. ST1 was found in 5 isolates of F. petroliphilum (FSSC) from blood that were recovered from hospitalized patients from 2007 to 2013 (BMT: 1 isolate, Hematology: 4 isolates), and 7 isolates of F. petroliphilum recovered from the air in 2012 (BMT: 6 isolates, Hematology: 1 isolate) (Table 2). STn1 (F. napiforme) was detected in one isolate from blood culture of a patient that was hospitalized in the hematology unit and in one isolate from the air. Both were isolated in 2013 from the same room during the time that the patient was hospitalized and showed a genetic relationship using MLST.
Table 2. Detailed information about ST1 (F. petroliphilum—FSSC) and STn1 (F. napiforme—FFSC) isolates.
| Sample | Source | Isolation date | Ward |
|---|---|---|---|
| ST1 (F. petroliphilum—FSSC) | |||
| 952 | Blood | 11/28/07 | Hematology |
| 1196 | Blood | 03/29/08 | BMT |
| 1549 | Blood | 12/09/10 | Hematology |
| 1750 | Blood | 06/03/11 | Hematology |
| 2020 | Blood | 06/26/13 | Hematology |
| F16 | Air | 03/05/12 | Hematology |
| F17-1 | Air | 03/29/12 | BMT |
| F17-2 | Air | 03/29/12 | BMT |
| F50 | Air | 10/10/12 | BMT |
| F51 | Air | 10/10/12 | BMT |
| F52 | Air | 10/10/12 | BMT |
| F54 | Air | 10/10/12 | BMT |
| STn1 (F. napiforme- FFSC) | |||
| 2008 | Blood | 11/27/13 | Hematology |
| F111 | Air | 03/21/13 | Hematology |
FSSC: F. solani species complex; FFSC: F. fujikuroi species complex; BMT: bone marrow transplant ward.
Discussion
Fusarium species are ubiquitous in the environment, whose spores can easily be carried by wind and rain, causing transmission and subsequent infection to humans. Our study showed, for the first time, a genetic relationship between Fusarium species isolated from indoor hospital air with the ones recovered in blood cultures of hematologic patients, suggesting that the air may be a potential source for fusariosis.
Air samplings were performed during one year in BMT and hematology units in our Clinical Hospital. The Clinical Hospital is located in an area surrounded by farms of soy and canola plantations, which may be a potential source of the Fusarium isolated in the hospital air once the species are mainly plant pathogens[1]. The hospital is placed on an area of tropical climate with dry winters and very warm rainy summers. Although climate conditions have been previously associated with the onset of crop disease by Fusarium[28, 29], the association between the weather and the development of fusariosis is poorly known. A peak of Fusarium spores count isolated from indoor hospital and outdoor air in Houston, Texas, USA, was found in the rainy summer of 1988 and 1989[30]. This peak correlated with the seasonal clustering of cases of fusariosis. However, we could not find a correlation between temperature or humidity and the frequency of Fusarium isolates recovered in the hospital air.
The recovery of Fusarium isolates in the BMT rooms might suggest that the laminar airflow systems were not sufficiently protecting our patients. As the hematology unit does not have controlled air, the higher number of isolates, and the species diversity of Fusarium might represent the outside air condition. We presumed that the recovery of a substantial amount of Fusarium isolates from the air could be due to the modified selective culture medium used for air sampling[18]. In most studies, Sabouraud Agar formulations were used in the air samplers, and as Aspergillus and others filamentous fungi are more prevalent in the air, they were first recovered than Fusarium in environmental surveillances[15, 31].
The identification of Fusarium to complex or species levels is very important not only to select an appropriate antifungal agent but also to clarify the epidemiology. In this study, we applied TEF1α DNA sequencing for identification of the genus Fusarium for strains recovered from the air (n = 104) and blood (n = 18). The identification of members of FSSC is of special importance because this complex is responsible for about 50% of human infections, and comprises the most virulent species[3]. In addition, FSSC are usually resistant to azoles in vitro and exhibit higher minimum inhibitory concentration for amphotericin B than other Fusarium species[32, 33].
The sequencing of the TEF1α locus classified the non-FSSC isolated from air into four complexes: FCSC, FFSC, FIESC and FOSC. The FOSC members are of special interest, because they are known as the second most common species in human fusariosis, representing approximately 20% of the cases. Surprisingly, in our study, only one isolate was recovered from the air, and no FOSC was identified from clinical samples. F. oxysporum isolates have been recovered from several hospital environmental sources, including water[34], shower, sink and bathroom swab[23], but not in the air, suggesting that maybe FOSC is more likely to be recovered from different environmental sources than air.
We used a MLST scheme based on partial sequencing of TEF1α, rDNA, RPB1 and RPB2 genes [20, 21, 23, 24, 26] for determining the clonality of Fusarium isolated from air and blood. New primers sequences were designed to cover regions and gaps of the RPB2 gene that were not properly sequenced by the primers previously reported. Previous studies reported the molecular relationship between clinical and environmental isolates from hospital water system, hospital surfaces and plumbing drains of external buildings[23, 30, 34, 35]. Revising the recent literature, there are not robust data implicating the air or other environment as potential sources of fusariosis, in hospital settings. Carlesse et al. [36] reported an outbreak of catheter-related fungemia caused by F. oxysporum but no environmental source was identified. Litvinov et al. [37] described an outbreak of invasive fusariosis in a children´s cancer hospital. They recovered Fusarium from the environment (water, air, swabs) of patients’ rooms, but no molecular correlation was done in this study. In the study performed by Edel-Hermann et al. [38] the authors described the clonal linage of F. oxysporum isolated from the tap water of different French hospitals, but no correlation was established with clinical samples. Short et al. [35] described the occurrence of a clonal distribution of the same sequencing typing of Fusarium spp. clinical isolates and in the plumbing drains of hospitals and other facilities.
To our knowledge, our study is the first one describing the identification of two clonal STs: Fusarium petroliferum and Fusarium napiforme isolated in the air and in blood of patients with fusariosis hospitalized in our hospital. In our study, we found ST1 as the most frequent ST belonging to FSSC (F. petroliphilum), and ST1 was identified in five of the clinical isolates and in seven isolates from air, indicating a possible environmental source of fusariosis. In addition, one F. napiforme was isolated from air sampling and the molecular typing showed the same ST for this environmental isolate and for one F. napiforme recovered from blood (STn1). F. napiforme was recovered from the patient´s blood culture and from the air of the same room in which the patient was hospitalized, in the hematology unit in 2013[17]. F. napiforme is a less frequent pathogen and very few cases were previously reported causing keratomycosis[39], hypersensitivity pneumonitis[40], and systemic infection in immunocompromised patients[17, 41].
The molecular typing revealed that F. petroliphilum and F. napiforme recovered in the air samplings were related to the ones that caused systemic diseases. In conclusion, systemic fusariosis may occur from the encounter of Fusarium spp. present in environmental sources, such as in the air of our hospital, and the susceptible patients hospitalized in BMT and hematology units. This study indicates that there is a need for effective surveillance of hospital environment quality control.
Supporting information
(DOCX)
(DOCX)
The long bars represents TEF1α (A), rDNA (B), RPB1 (C) and RPB2 (D) genes, and the black boxes and numbers above represents the amino acids motifs. The positions of the primers and base pairs (bp) amplified are represented below each gene (right arrow: forward primer; left arrow: reverse primer).
(DOCX)
It was generated by maximum likelihood (ML) from 77 –TEF1α sequences, 578 characters, percentages of 1,000 bootstrap-replications of MEGA6-maximum likelihood (ML). The tree was rooted with the F. oxysporum CBS 463.61.
(DOCX)
It was generated by maximum likelihood (ML) from 37 –EF1α sequences, 570 characters, percentages of 1,000 bootstrap-replications of MEGA6-maximum likelihood (ML). The tree was rooted with Fusarium staphyleae NRRL 22316.
(DOCX)
It was generated by maximum likelihood (ML) from 43 –TEF1α sequences, 556 characters, percentages of 1,000 bootstrap-replications of MEGA6-maximum likelihood (ML). The tree was rooted with Fusarium sporotrichioides NRRL 52934. Abbreviations–FIESC: F. incarnatum-equiseti species complex. FCSC: F. chlamydosporum species complex.
(DOCX)
Acknowledgments
Financial support
This project was supported by grants from the collaborative research project: Science and Technology Research Partnership for Sustainable Development, Japan (SATREPS) and University of Campinas, Brazil, No. 02P-29548-09 and Fundação de Amparo à Pesquisa do Estado de Sao Paulo, FAPESP No. 2012/51158-0. We would like to thank Dr. Haruhisa Suga (Life Science Research Center, Gifu University, Gifu, Japan) for kindly providing the sequences of HS392 and HS393 primers.
We gratefully appreciated the substation collaborations of: Mrs. Pamella Stivanelli-Ranal during DNA sequencing and Mr. Roger Timothy Rentfrow for the English revision of the manuscript.
Data Availability
All relevant data are within the paper and in the Supporting Information files.
Funding Statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de Sao Paulo, FAPESP No. 2012/51158-0, and by the Science and Technology Research Partnership for Sustainable Development, Japan (SATREPS) and University of Campinas, Brazil, No. 02P-29548-09.
References
- 1.Zhang N, O'Donnell K, Sutton DA, Nalim FA, Summerbell RC, Padhye AA, et al. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J Clin Microbiol. 2006;44(6):2186–90. doi: 10.1128/JCM.00120-06 ; PubMed Central PMCID: PMCPMC1489407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chilaka CA, De Boevre M, Atanda OO, De Saeger S. The Status of Fusarium Mycotoxins in Sub-Saharan Africa: A Review of Emerging Trends and Post-Harvest Mitigation Strategies towards Food Control. Toxins (Basel). 2017;9(1). doi: 10.3390/toxins9010019 ; PubMed Central PMCID: PMCPMC5308251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nucci M, Anaissie E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007;20(4):695–704. doi: 10.1128/CMR.00014-07 ; PubMed Central PMCID: PMCPMC2176050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muhammed M, Anagnostou T, Desalermos A, Kourkoumpetis TK, Carneiro HA, Glavis-Bloom J, et al. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine (Baltimore). 2013;92(6):305–16. doi: 10.1097/MD.0000000000000008 ; PubMed Central PMCID: PMCPMC4553992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guevara-Suarez M, Cano-Lira JF, de Garcia MC, Sopo L, De Bedout C, Cano LE, et al. Genotyping of Fusarium Isolates from Onychomycoses in Colombia: Detection of Two New Species Within the Fusarium solani Species Complex and In Vitro Antifungal Susceptibility Testing. Mycopathologia. 2016;181(3–4):165–74. doi: 10.1007/s11046-016-9983-9 . [DOI] [PubMed] [Google Scholar]
- 6.Doczi I, Gyetvai T, Kredics L, Nagy E. Involvement of Fusarium spp. in fungal keratitis. Clin Microbiol Infect. 2004;10(9):773–6. doi: 10.1111/j.1469-0691.2004.00909.x . [DOI] [PubMed] [Google Scholar]
- 7.Mosquera Gordillo MA, Baron Cano N, Garralda Luquin A, Lopez Gutierrez C, Mengual Verdu E, Trujillo Cabrera G, et al. Keratitis secondary to Fusarium spp. in Spain 2012–2014. Arch Soc Esp Oftalmol. 2017. doi: 10.1016/j.oftal.2017.08.005 . [DOI] [PubMed] [Google Scholar]
- 8.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36(1):1–53. doi: 10.3109/10408410903241444 . [DOI] [PubMed] [Google Scholar]
- 9.Lass-Florl C, Cuenca-Estrella M. Changes in the epidemiological landscape of invasive mould infections and disease. J Antimicrob Chemother. 2017;72(suppl_1):i5–i11. doi: 10.1093/jac/dkx028 . [DOI] [PubMed] [Google Scholar]
- 10.Nucci M, Marr KA, Queiroz-Telles F, Martins CA, Trabasso P, Costa S, et al. Fusarium infection in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2004;38(9):1237–42. doi: 10.1086/383319 . [DOI] [PubMed] [Google Scholar]
- 11.Nucci M, Garnica M, Gloria AB, Lehugeur DS, Dias VC, Palma LC, et al. Invasive fungal diseases in haematopoietic cell transplant recipients and in patients with acute myeloid leukaemia or myelodysplasia in Brazil. Clin Microbiol Infect. 2013;19(8):745–51. doi: 10.1111/1469-0691.12002 . [DOI] [PubMed] [Google Scholar]
- 12.Al-Hatmi AM, van Diepeningen AD, Curfs-Breuker I, de Hoog GS, Meis JF. Specific antifungal susceptibility profiles of opportunists in the Fusarium fujikuroi complex. J Antimicrob Chemother. 2015;70(4):1068–71. doi: 10.1093/jac/dku505 . [DOI] [PubMed] [Google Scholar]
- 13.Alastruey-Izquierdo A, Cuenca-Estrella M, Monzon A, Mellado E, Rodriguez-Tudela JL. Antifungal susceptibility profile of clinical Fusarium spp. isolates identified by molecular methods. J Antimicrob Chemother. 2008;61(4):805–9. doi: 10.1093/jac/dkn022 . [DOI] [PubMed] [Google Scholar]
- 14.Center for International B, Marrow Transplant R, National Marrow Donor P, European B, Marrow Transplant G, American Society of B, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective. Bone Marrow Transplant. 2009;44(8):453–558. . [PubMed] [Google Scholar]
- 15.Hahn T, Cummings KM, Michalek AM, Lipman BJ, Segal BH, McCarthy PL Jr. Efficacy of high-efficiency particulate air filtration in preventing aspergillosis in immunocompromised patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2002;23(9):525–31. doi: 10.1086/502101 . [DOI] [PubMed] [Google Scholar]
- 16.Lacaz CS, Porto E, Martins JEC, Heins-Vaccari EM, Takahashi de Melo N. Tratado de micologia médica. 9 ed Sao Paulo: Sarvier; 2002. 1104 p. [Google Scholar]
- 17.de Souza M, Matsuzawa T, Lyra L, Busso-Lopes AF, Gonoi T, Schreiber AZ, et al. Fusarium napiforme systemic infection: case report with molecular characterization and antifungal susceptibility tests. Springerplus. 2014;3:492 doi: 10.1186/2193-1801-3-492 ; PubMed Central PMCID: PMCPMC4159480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.H K. A new selective medium for isolating Fusarium from natural soil. Proceedings of the American Phytopathological Society. 1976;3:221. [Google Scholar]
- 19.A C. Maintenance and cultivation of the common pathogenic fungi of man in sterile distilled water. Am J Trop Med Hyg. 1967;70:181. [Google Scholar]
- 20.Muraosa Y, Schreiber AZ, Trabasso P, Matsuzawa T, Taguchi H, Moretti ML, et al. Development of cycling probe-based real-time PCR system to detect Fusarium species and Fusarium solani species complex (FSSC). Int J Med Microbiol. 2014;304(3–4):505–11. doi: 10.1016/j.ijmm.2014.03.001 . [DOI] [PubMed] [Google Scholar]
- 21.O'Donnell K, Kistler HC, Cigelnik E, Ploetz RC. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci U S A. 1998;95(5):2044–9. ; PubMed Central PMCID: PMCPMC19243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–9. doi: 10.1093/molbev/mst197 ; PubMed Central PMCID: PMCPMC3840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheel CM, Hurst SF, Barreiros G, Akiti T, Nucci M, Balajee SA. Molecular analyses of Fusarium isolates recovered from a cluster of invasive mold infections in a Brazilian hospital. BMC Infect Dis. 2013;13:49 doi: 10.1186/1471-2334-13-49 ; PubMed Central PMCID: PMCPMC3579725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Donnell K, Sutton DA, Rinaldi MG, Gueidan C, Crous PW, Geiser DM. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol. 2009;47(12):3851–61. doi: 10.1128/JCM.01616-09 ; PubMed Central PMCID: PMCPMC2786663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilak R, Singh A, Maurya OP, Chandra A, Tilak V, Gulati AK. Mycotic keratitis in India: a five-year retrospective study. J Infect Dev Ctries. 2010;4(3):171–4. . [DOI] [PubMed] [Google Scholar]
- 26.Liu YJ, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol Biol Evol. 1999;16(12):1799–808. doi: 10.1093/oxfordjournals.molbev.a026092 . [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell K, Sutton DA, Rinaldi MG, Sarver BA, Balajee SA, Schroers HJ, et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol. 2010;48(10):3708–18. doi: 10.1128/JCM.00989-10 ; PubMed Central PMCID: PMCPMC2953079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smiley RW. Water and temperature parameters associated with winter wheat diseases caused by soilborne pathogens. Plant Dis. 2009;93(1):73–80. [DOI] [PubMed] [Google Scholar]
- 29.Moya-Elizondo E, Rew LJ, Jacobsen JB, Hogg AC, Dyer AT. Distribution and prevalence of Fusarium crown rot and common root rot pathogens of wheat in Montana. Plant Dis. 2011;95(9):1099–108. [DOI] [PubMed] [Google Scholar]
- 30.Raad I, Tarrand J, Hanna H, Albitar M, Janssen E, Boktour M, et al. Epidemiology, molecular mycology, and environmental sources of Fusarium infection in patients with cancer. Infect Control Hosp Epidemiol. 2002;23(9):532–7. doi: 10.1086/502102 . [DOI] [PubMed] [Google Scholar]
- 31.Panagopoulou P, Filioti J, Petrikkos G, Giakouppi P, Anatoliotaki M, Farmaki E, et al. Environmental surveillance of filamentous fungi in three tertiary care hospitals in Greece. J Hosp Infect. 2002;52(3):185–91. . [DOI] [PubMed] [Google Scholar]
- 32.Cuenca-Estrella M, Gomez-Lopez A, Mellado E, Buitrago MJ, Monzon A, Rodriguez-Tudela JL. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob Agents Chemother. 2006;50(3):917–21. doi: 10.1128/AAC.50.3.917-921.2006 ; PubMed Central PMCID: PMCPMC1426453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arikan S, Lozano-Chiu M, Paetznick V, Nangia S, Rex JH. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J Clin Microbiol. 1999;37(12):3946–51. ; PubMed Central PMCID: PMCPMC85852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anaissie EJ, Kuchar RT, Rex JH, Francesconi A, Kasai M, Muller FM, et al. Fusariosis associated with pathogenic fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin Infect Dis. 2001;33(11):1871–8. doi: 10.1086/324501 . [DOI] [PubMed] [Google Scholar]
- 35.Short DP, O'Donnell K, Zhang N, Juba JH, Geiser DM. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J Clin Microbiol. 2011;49(12):4264–72. doi: 10.1128/JCM.05468-11 ; PubMed Central PMCID: PMCPMC3232942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlesse F, Amaral AC, Goncalves SS, Xafranski H, Lee MM, Zecchin V, et al. Outbreak of Fusarium oxysporum infections in children with cancer: an experience with 7 episodes of catheter-related fungemia. Antimicrob Resist Infect Control. 2017;6:93 doi: 10.1186/s13756-017-0247-3 ; PubMed Central PMCID: PMCPMC5588724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Litvinov N, da Silva MT, van der Heijden IM, Graca MG, Marques de Oliveira L, Fu L, et al. An outbreak of invasive fusariosis in a children's cancer hospital. Clin Microbiol Infect. 2015;21(3):268 e1-7. doi: 10.1016/j.cmi.2014.09.004 . [DOI] [PubMed] [Google Scholar]
- 38.Edel-Hermann V, Sautour M, Gautheron N, Laurent J, Aho S, Bonnin A, et al. A Clonal Lineage of Fusarium oxysporum Circulates in the Tap Water of Different French Hospitals. Appl Environ Microbiol. 2016;82(21):6483–9. doi: 10.1128/AEM.01939-16 ; PubMed Central PMCID: PMCPMC5066365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Homa M, Shobana CS, Singh YR, Manikandan P, Selvam KP, Kredics L, et al. Fusarium keratitis in South India: causative agents, their antifungal susceptibilities and a rapid identification method for the Fusarium solani species complex. Mycoses. 2013;56(5):501–11. doi: 10.1111/myc.12062 . [DOI] [PubMed] [Google Scholar]
- 40.Lee SK, Kim SS, Nahm DH, Park HS, Oh YJ, Park KJ, et al. Hypersensitivity pneumonitis caused by Fusarium napiforme in a home environment. Allergy. 2000;55(12):1190–3. . [DOI] [PubMed] [Google Scholar]
- 41.Melcher GP, McGough DA, Fothergill AW, Norris C, Rinaldi MG. Disseminated hyalohyphomycosis caused by a novel human pathogen, Fusarium napiforme. J Clin Microbiol. 1993;31(6):1461–7. ; PubMed Central PMCID: PMCPMC265562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
The long bars represents TEF1α (A), rDNA (B), RPB1 (C) and RPB2 (D) genes, and the black boxes and numbers above represents the amino acids motifs. The positions of the primers and base pairs (bp) amplified are represented below each gene (right arrow: forward primer; left arrow: reverse primer).
(DOCX)
It was generated by maximum likelihood (ML) from 77 –TEF1α sequences, 578 characters, percentages of 1,000 bootstrap-replications of MEGA6-maximum likelihood (ML). The tree was rooted with the F. oxysporum CBS 463.61.
(DOCX)
It was generated by maximum likelihood (ML) from 37 –EF1α sequences, 570 characters, percentages of 1,000 bootstrap-replications of MEGA6-maximum likelihood (ML). The tree was rooted with Fusarium staphyleae NRRL 22316.
(DOCX)
It was generated by maximum likelihood (ML) from 43 –TEF1α sequences, 556 characters, percentages of 1,000 bootstrap-replications of MEGA6-maximum likelihood (ML). The tree was rooted with Fusarium sporotrichioides NRRL 52934. Abbreviations–FIESC: F. incarnatum-equiseti species complex. FCSC: F. chlamydosporum species complex.
(DOCX)
Data Availability Statement
All relevant data are within the paper and in the Supporting Information files.