Abstract
Purpose
Some cancers are largely preventable through modification of certain behavioral risk factors and preventive screening, even among those with a family history of cancer. This study examined the associations between: (1) family cancer history and cancer screening; (2) family history and cancer preventive lifestyle behaviors; and, (3) cancer screening and lifestyle behaviors.
Methods
Data were from the 2009 California Health Interview Survey (n=12,603). Outcomes included screening for breast cancer (BC) and colorectal cancer (CRC) and six cancer preventive lifestyle behaviors, based on World Cancer Research Fund recommendations. Multivariate logistic regression analyses, stratified by gender and race-ethnicity, examined associations. Predicted probabilities of cancer screening by family cancer history, race-ethnicity and sex were computed.
Results
Family history of site-specific cancer—CRC for men and women, and BC for women—was associated with higher probability of cancer screening for most groups, especially for CRC, but was largely unrelated to other lifestyle behaviors. In the few cases in which family history was significantly associated with lifestyle—e.g., physical activity among White and Latino males, smoking among White and Asian females—individuals with a family history had lower odds of adherence to recommendations than those with no family history. Greater overall adherence to lifestyle recommendations was associated with higher odds of up-to-date CRC screening among White and Asian males, and lower odds among Asian females (no significant association with BC screening); this relationship did not vary by family cancer history.
Conclusions
The fact that family history of cancer is not associated with better lifestyle behaviors may reflect shared behavioral risks within families, or the lack of knowledge about how certain lifestyle behaviors impact personal cancer risk. Findings can inform interventions aimed at lifestyle behavioral modification for individuals at increased cancer risk due to family history.
Keywords: family cancer history, cancer prevention, health behaviors, colorectal cancer, breast cancer
Introduction
Most cancers are largely preventable. An estimated 30% of cancers can be prevented by avoiding exposure to tobacco [1]. An additional 30–40% of cancers can be prevented by modifying other lifestyle factors such as physical activity and diet [2]. For some cancers, mortality can further be reduced through preventive screening at recommended age-specific intervals, designed to detect cancer at an earlier, potentially more treatable stage. Following cancer preventive lifestyle and screening recommendations is especially important for populations that are at above-average risk due to a family history of cancer. Breast cancer (BC) and colorectal cancer (CRC) have the second and third highest familial proportions, with nearly 13% of cases having a family member with concordant cancer [3]. Yet even among those with a family history of cancer, lifestyle behaviors are associated with risk of the disease [4]. Family history of cancer may reflect both shared genetic and behavioral/environmental risks [5], therefore it is important to understand how family history is associated with cancer preventive behaviors in families. This study examines associations between self-reported family history of cancer (BC and CRC), cancer screening, and other cancer-preventive lifestyle behaviors.
The role of family history of cancer in influencing cancer preventive behaviors is unclear. Several intervention studies, aimed at increasing cancer screening rates, have targeted individuals with a family history and used personalized risk notifications designed to increase perceived risk as part of the intervention [6–8]. However, few studies have examined whether family history is associated with preventive behaviors at the population level in the absence of a specific intervention. A study in Spain found little indication that family history of CRC was related to increased adherence to cancer preventive behaviors [9], but there is a dearth of evidence from the United States on how family history is associated with cancer screening and cancer preventive lifestyle behaviors. A recent US study of Californians found that family history of CRC was associated with higher likelihood of CRC screening among Whites, but not Latinos [10]. Another study found that family history was associated with cancer screening, but not cancer preventive lifestyle behaviors [11]. Similarly, a U.S. study found that women with a family history of BC were more likely to undertake medical preventive behaviors (such as screening and use of anti-estrogenic agents), but not lifestyle behaviors [12]. Thus, there is ongoing debate about how knowledge of family history may influence individuals’ lifestyle behaviors [5, 13, 14].
Moreover, some research suggests that there may be “spillover” between cancer preventive behaviors; for example, those who avoid tobacco use are also more likely to engage in physical activity [15, 16]. However, few studies examine health-related lifestyle behaviors and screening simultaneously to address whether engaging in certain cancer preventive behaviors (e.g., screening) is associated with greater adherence to other cancer prevention recommendations (e.g., cancer-preventive lifestyle). Understanding how these two types of cancer prevention behaviors are associated, and whether these associations vary for those with a family history, could yield insight into more effective strategies to improve cancer prevention efforts among those at highest risk, including those with a family history of cancer.
This study examined an ethnically-diverse, population-based sample to assess adherence to CRC and BC screening guidelines from the US Preventive Services Task Force (USPSTF), and cancer preventive lifestyle recommendations from the World Cancer Research Fund-American Institute for Cancer Research (WCRF-AICR) [2]. The lifestyle measures we examined included smoking, body mass index (BMI), physical activity, consumption of foods and drinks that promote weight gain, consumption of fruits and vegetables, and alcohol use. The research questions were: 1. Are individuals with a family history of cancer more likely to be up-to-date with cancer screening recommendations compared to those with no family history? 2. Are individuals with a family history of cancer more likely to engage in cancer preventive lifestyle behaviors? 3. Is adherence to cancer preventive behaviors associated with a higher likelihood of cancer screening, and does this relationship vary by family cancer history? This study goes beyond existing literature by considering how family history is associated with both screening and cancer preventive behaviors separately by sex and race-ethnicity, and how screening is associated with cancer preventive behaviors.
Methods
Data and Sample
Data were from the 2009 California Health Interview Survey (CHIS) [17], a random-digit-dial (RDD) survey of landline and cellular telephones. The CHIS sample is considered to be representative of the California population when sampling weights are used to account for the sampling design and to adjust for differential non-response. The 2009 overall response rate (36.1%) is comparable to other California surveys [18], and to national RDD surveys [19]. Respondents were asked detailed questions, including about their own health status and health behaviors, cancer screening history, and whether their biological parents, siblings, or children had cancer of any kind (follow-up questions probed about specific cancers). Analyses were limited to non-Latino Whites, Latinos, Blacks and Asians ages 40 to 75 (n=12,603); other race/ethnicity groups were excluded because of small cell sizes.
Dependent Measures
All measures were self-reported. USPSTF recommendations on CRC and BC screening for average-risk individuals were used [20]; although USPSTF recommends that individuals with a family history be screened at even younger ages, no specific guidelines are provided, therefore only average-risk recommendations were used. Respondents ages 50–75 were defined as up-to-date with CRC screening if they reported one of the following: fecal occult blood test (FOBT) within the past year; sigmoidoscopy within past 5 years, combined with FOBT within past 2 years; or colonoscopy within past 10 years. While guidelines specify sigmoidoscopy within 5 years combined with FOBT every 3 years, FOBT response categories in CHIS were: within past year, 1–2 years ago, 2–5 years ago, and more than 5 years ago. We chose to use past 2 years, which may slightly underestimate the number up-to-date with CRC screening. Women ages 40–75 were considered up-to-date with BC screening if they reported having a mammogram within the past 2 years, based on then-current USPSTF recommendations. The USPSTF’s change in recommended starting age from 40 to 50 in 2009 was unlikely to have affected many survey responses [21].
The following measures of health behaviors were included, based on WCRF-AICR recommendations [2]: smoking, BMI, physical activity,1 consumption of foods and drinks that promote weight gain, consumption of fruits and vegetables, and alcohol use. Respondents were asked about: smoking habits; height and weight (used to calculate BMI); number of days and amount of time spent engaging in leisure-time vigorous and moderate physical activity (in past seven days); past month consumption of sugar-sweetened beverages (including soda, sports or energy drinks, sweetened fruit juices or coffee/tea); past week consumption of fast food; past month consumption of fruits and vegetables (not including fried potatoes); past year consumption of alcohol. All behaviors were coded to be as consistent as possible with WCRF-AICR recommendations: 0 = no adherence to recommendation, 1 = partial adherence, 2 = full adherence (see Supplemental Table 1 for definitions). The measures were summed to create a Lifestyle Adherence Scale ranging from 0 to 12, with 12 indicating full adherence to these six recommendations.
Independent Measures
Family history of any cancer and site-specific family history of BC or CRC were examined. A respondent was considered to have any family history of cancer if he/she answered “yes” to the question, “Did your biological father or mother, full brothers or sisters, or biological sons or daughters ever have cancer of any kind?” Such respondents were then asked what kinds of cancer. Individuals who reported having a blood relative with CRC were considered to have a CRC family history, and females who reported having a mother or sister with BC were considered to have a BC family history.
Covariates included: age, sex, marital status, education (less than high school, high school diploma, college degree), family income (below/above federal poverty line), currently employed (yes/no), home ownership (yes/no), continuous health insurance coverage past 12 months (yes/no), rural/urban residence, doctor visit in the past 12 months (yes/no), usual source of care (yes/no), English proficiency (speaks English well/very well vs. not well/not at all), and nativity (foreign-born/US-born).
Data Analysis
All analyses were conducted in Stata version 12 [22], weighted to account for the complex sampling design, and were stratified by sex and race-ethnicity. Chi-square and t-tests were used to assess differences in sociodemographic characteristics between individuals with and without a family history of cancer (Table 1). Differences between individuals with and without a family history in the prevalence of up-to-date screening and the six cancer preventive lifestyle behaviors were tested using chi square tests (Table 2). We conducted multivariate logistic regressions predicting CRC screening (males and females), and BC screening (females), including an interaction between race-ethnicity and family history of site-specific cancer; from those regression equations, we calculated predicted probabilities of screening by sex, race-ethnicity and family history, holding covariates constant at their means (Figure 1). We examined whether family history was associated with lifestyle behaviors using Poisson regression for the Lifestyle Adherence Scale and ordered logistic regression for other outcomes (Table 3). We examined whether each lifestyle behavior was associated with cancer screening by regressing screening on each lifestyle behavior separately (Table 4). Adjusted Wald tests examined whether the odds associated with full adherence differed significantly from those for partial adherence. All regression models controlled for the aforementioned covariates.
Table 1.
Sociodemographic Characteristics of Californians (Ages 40–75) by Sex, Race-Ethnicity, and Self-reported Family History of Cancer: Weighted Prevalence (%)
| Males | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Latino | Black | Asian | |||||||||
| FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
|
| Sample size (n) | 5,043 | 3,860 | 650 | 1,188 | 189 | 286 | 422 | 965 | ||||
| Age, Mean (t-test sig.) | 56.3 | 53.7 | *** | 53.3 | 50.6 | *** | 54.8 | 51.5 | † | 53.7 | 53.3 | |
| Not married | 27.7 | 31.4 | † | 34.2 | 24.3 | † | 48.6 | 54.8 | 30.3 | 11.8 | ** | |
| Family income below FPLa | 3.0 | 6.6 | *** | 15.8 | 32.3 | *** | 15.7 | 24.7 | 19.0 | 10.6 | ||
| Unemployed | 30.9 | 26.2 | * | 25.7 | 27.2 | 43.5 | 34.2 | 29.1 | 29.1 | |||
| Education | † | *** | † | |||||||||
| Up to 12th grade, no HS diploma | 3.3 | 4.9 | 36.9 | 56.6 | 9.4 | 25.2 | 16.4 | 12.7 | ||||
| High school graduate | 44.9 | 46.6 | 47.8 | 33.5 | 71.1 | 50.8 | 23.7 | 32.5 | ||||
| College or more | 51.8 | 48.5 | 15.3 | 9.9 | 19.5 | 23.9 | 59.9 | 54.8 | ||||
| Rural residence | 15.3 | 16.3 | 7.4 | 7.2 | 0.6 | 1.5 | 3.8 | 2.8 | ||||
| Does not own home | 19.2 | 24.8 | ** | 36.9 | 44.9 | † | 42.5 | 51.2 | 19.8 | 37.2 | ** | |
| Uninsured, past 12 mos. | 7.1 | 8.7 | 18.1 | 25.5 | † | 11.5 | 26.4 | † | 4.5 | 7.5 | † | |
| No doctor visit, past 12 mos. | 15.6 | 17.8 | 19.3 | 26.6 | * | 18.8 | 14.7 | 12.3 | 14.4 | |||
| No usual source of care | 10.4 | 9.3 | 17.6 | 28.5 | ** | 16.0 | 29.7 | 12.3 | 12.5 | |||
| Foreign-born | 7.9 | 10.7 | † | 56.9 | 77.6 | *** | 3.8 | 12.9 | *** | 67.8 | 86.3 | ** |
| Limited English Proficiencyb | 0.1 | 0.7 | *** | 34.3 | 54.6 | *** | 0.2 | 0.0 | 18.1 | 32.0 | *** | |
| Females | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Latino | Black | Asian | |||||||||
| FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
|
| Sample size (n) | 7,595 | 4,971 | 1,083 | 1,647 | 413 | 410 | 658 | 1,140 | ||||
| Age, Mean (t-test sig.) | 56.1 | 53.8 | *** | 53.8 | 51.5 | ** | 57.0 | 51.8 | *** | 55.8 | 52.2 | *** |
| Not married | 34.6 | 34.6 | 41.3 | 36.9 | 55.6 | 59.5 | 25.9 | 23.3 | ||||
| Family income below FPL | 4.7 | 8.3 | † | 22.6 | 32.5 | ** | 11.6 | 16.6 | 6.4 | 10.4 | ||
| Unemployed | 41.9 | 41.0 | 48.3 | 45.1 | 50.1 | 37.2 | * | 36.3 | 37.0 | |||
| Education | † | ** | ||||||||||
| Up to 12th grade, no HS diploma | 3.4 | 5.3 | 35.6 | 47.6 | 9.2 | 7.1 | 9.2 | 13.7 | ||||
| High school graduate | 53.0 | 51.7 | 49.9 | 39.2 | 55.1 | 57.2 | 33.6 | 31.4 | ||||
| College or more | 43.6 | 43.0 | 14.5 | 13.3 | 35.8 | 35.7 | 57.2 | 54.9 | ||||
| Rural residence | 16.3 | 15.9 | 6.9 | 7.5 | 2.9 | 1.3 | † | 2.8 | 2.5 | |||
| Does not own home | 17.6 | 22.3 | * | 34.8 | 40.1 | † | 44.0 | 43.9 | 23.6 | 26.5 | ||
| Uninsured, past 12 mos. | 4.9 | 8.5 | † | 18.9 | 21.5 | 7.7 | 5.9 | 6.3 | 8.9 | |||
| No doctor visit, past 12 mos. | 7.8 | 12.6 | * | 10.6 | 9.7 | 6.8 | 8.0 | 7.9 | 13.5 | * | ||
| No usual source of care | 4.2 | 6.2 | * | 13.6 | 16.2 | 3.7 | 8.3 | * | 10.9 | 9.5 | ||
| Foreign-born | 8.6 | 11.0 | † | 56.3 | 69.4 | *** | 11.6 | 9.7 | 82.2 | 88.7 | * | |
| Limited English Proficiencyb | 0.2 | 0.4 | 40.5 | 51.7 | ** | 0.3 | 0.4 | 25.5 | 28.5 | |||
Notes: Data come from the California Health Interview Survey (2009) and were weighted to represent the state population. FMH denotes self-reported family history of cancer.
p < .001
p < 0.01
p < 0.05
p < 0.10 (two-tailed test).
FPL, Family income below poverty line
Speaks English not well/not at all
Notes: Data come from the California Health Interview Survey (2009) and were weighted to represent the state population. FMH denotes self-reported family history of cancer.
p < .001
p < 0.01
p < 0.05
p < 0.10 (two-tailed test).
FPL, Family income below poverty line
Speaks English not well/not at all
Table 2.
Cancer Preventive Behaviors of Californians (Ages 40–75) by Sex, Race-ethnicity, and Family History of Cancer: Weighted Prevalence (%)
| Males | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Latino | Black | Asian | |||||||||
| FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
|
| Screening | ||||||||||||
| CRC screening (up-to-date, ages 50–75)a | 68.8 | 65.5 | 54.4 | 45.6 | 53.2 | 50.0 | 67.8 | 53.2 | ** | |||
| Health Behaviors and Body Mass Index | ||||||||||||
| Mean, Lifestyle Adherence Scale (0–12)b | 5.5 | 5.6 | 4.7 | 4.8 | 4.8 | 5.3 | 5.8 | 6.1 | ||||
| Smoking Status | * | |||||||||||
| 0 (Current) | 13.3 | 13.5 | 18.3 | 17.8 | 16.4 | 15.4 | 13.8 | 15.4 | ||||
| 1 (Former) | 38.2 | 32.8 | 34.7 | 31.5 | 35.2 | 26.3 | 32.1 | 29.8 | ||||
| 2 (Never) | 48.6 | 53.7 | 47.1 | 50.7 | 48.4 | 58.2 | 54.2 | 54.8 | ||||
| Physical Activity, moderate-equivalent min. | † | † | ||||||||||
| 0 (<150 minutes/wk) | 56.4 | 51.9 | 70.8 | 61.9 | 71.2 | 59.1 | 63.6 | 72.0 | ||||
| 1 (150–299 min./wk) | 13.4 | 15.2 | 9.8 | 15.6 | 12.0 | 8.5 | 15.4 | 11.7 | ||||
| 2 (300+ min./week) | 30.1 | 32.8 | 19.4 | 22.5 | 16.7 | 32.3 | 21.0 | 16.3 | ||||
| Foods that promote weight gainc | * | |||||||||||
| 0 (no adherence) | 23.2 | 24.2 | 33.4 | 41.1 | 25.8 | 42.5 | 25.0 | 24.2 | ||||
| 1 (partial adherence) | 48.4 | 49.8 | 51.6 | 38.7 | 59.5 | 46.1 | 43.0 | 43.3 | ||||
| 2 (full adherence) | 28.4 | 26.0 | 15.0 | 20.2 | 14.7 | 11.5 | 32.0 | 32.4 | ||||
| Fruit/vegetable consumption | ** | |||||||||||
| 0 (0–1.9 servings/day) | 56.9 | 56.5 | 65.8 | 66.8 | 80.6 | 76.8 | 58.6 | 51.9 | ||||
| 1 (2–4.9 servings/day) | 37.2 | 40.0 | 31.0 | 30.7 | 17.4 | 21.3 | 40.0 | 44.8 | ||||
| 2 (5+ servings/day) | 5.9 | 3.5 | 3.2 | 2.5 | 2.0 | 1.9 | 1.4 | 3.4 | ||||
| Alcohol consumption, past 12 mos. | ** | |||||||||||
| 0 (Binge at least once) | 31.4 | 33.8 | 33.0 | 38.2 | 28.0 | 16.1 | 31.6 | 12.2 | ||||
| 1 (Some, no binge) | 49.3 | 47.8 | 39.0 | 30.4 | 48.2 | 55.7 | 44.5 | 50.7 | ||||
| 2 (None) | 19.4 | 18.4 | 27.9 | 31.4 | 23.8 | 28.2 | 23.9 | 37.1 | ||||
| Body mass index | † | † | ||||||||||
| 0 (<18.5 or ≥30kg/m2) | 29.0 | 26.3 | 36.8 | 34.5 | 31.2 | 23.9 | 11.0 | 9.7 | ||||
| 1 (25–29.99kg/m2) | 44.6 | 43.3 | 45.1 | 53.0 | 36.9 | 43.7 | 41.5 | 36.8 | ||||
| 2 (18.5–24.99kg/m2) | 26.4 | 30.4 | 18.1 | 12.6 | 31.9 | 32.4 | 47.5 | 53.5 | ||||
| Females | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Latino | Black | Asian | |||||||||
| FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
FMH | No FMH |
χ2 sig |
|
| Screening | ||||||||||||
| CRC screening (up-to-date, ages 50–75)a | 69.2 | 62.6 | ** | 57.4 | 56.0 | 66.6 | 66.8 | 63.7 | 56.0 | |||
| BC screening (up-to-date, ages 40–75)d | 91.0 | 87.1 | † | 90.0 | 90.7 | 91.3 | 91.8 | 87.5 | 90.3 | |||
| Health Behaviors and Body Mass Index | ||||||||||||
| Mean, Lifestyle Adherence Scale (0–12)b | 6.1 | 6.1 | 5.8 | 5.8 | 5.6 | 5.4 | 7.2 | 7.3 | ||||
| Smoking Status | ** | * | * | |||||||||
| 0 (Current) | 12.6 | 12.2 | 7.8 | 7.4 | 20.4 | 18.7 | 4.0 | 2.2 | ||||
| 1 (Former) | 33.5 | 28.5 | 19.6 | 15.1 | 25.5 | 23.7 | 10.9 | 5.9 | ||||
| 2 (Never) | 53.9 | 59.3 | 72.5 | 77.5 | 54.1 | 57.6 | 85.1 | 91.9 | ||||
| Physical Activity, moderate-equivalent min. | † | |||||||||||
| 0 (<150 minutes/wk) | 64.1 | 65.1 | 73.3 | 76.9 | 65.9 | 73.3 | 71.0 | 70.7 | ||||
| 1 (150–299 min./wk) | 13.8 | 12.3 | 12.7 | 9.2 | 20.7 | 9.5 | 15.9 | 12.3 | ||||
| 2 (300+ min./week) | 22.2 | 22.7 | 14.0 | 13.9 | 13.4 | 17.2 | 13.1 | 17.0 | ||||
| Foods that promote weight gainc | ||||||||||||
| 0 (no adherence) | 16.3 | 14.6 | 27.4 | 29.8 | 29.7 | 26.5 | 20.4 | 20.9 | ||||
| 1 (partial adherence) | 47.3 | 49.7 | 48.6 | 48 | 44.1 | 49.1 | 46.7 | 40.2 | ||||
| 2 (full adherence) | 36.5 | 35.7 | 24 | 22.2 | 26.2 | 24.4 | 32.9 | 38.9 | ||||
| Fruit/vegetable consumption | * | |||||||||||
| 0 (0–1.9 servings/day) | 39.2 | 44.0 | 46.9 | 51.2 | 44.8 | 44.8 | 29.4 | 35.1 | ||||
| 1 (2–4.9 servings/day) | 49.7 | 45.6 | 46.8 | 41.2 | 49.5 | 47.3 | 62.5 | 55.4 | ||||
| 2 (5+ servings/day) | 11.2 | 10.4 | 6.3 | 7.6 | 5.7 | 7.9 | 8.1 | 9.5 | ||||
| Alcohol consumption, past 12 mos. | * | * | ||||||||||
| 0 (Binge at least once) | 21.5 | 26.3 | 13.9 | 15.5 | 10.5 | 12.0 | 5.3 | 6.7 | ||||
| 1 (Some, no binge) | 57.0 | 52.5 | 44.7 | 36.1 | 46.7 | 54.4 | 41.0 | 35.5 | ||||
| 2 (None) | 21.5 | 21.2 | 41.4 | 48.4 | 42.8 | 33.6 | 53.7 | 57.8 | ||||
| Body mass index | ||||||||||||
| 0 (<18.5 or ≥30kg/m2) | 25.2 | 23.5 | 39.9 | 35.2 | 36.5 | 39.7 | 8.1 | 10.2 | ||||
| 1 (25–29.99kg/m2) | 30.8 | 30.6 | 30.9 | 35.2 | 39.0 | 32.2 | 24.3 | 25.7 | ||||
| 2 (18.5–24.99kg/m2) | 44.0 | 45.9 | 29.1 | 29.5 | 24.5 | 28.1 | 67.5 | 64.1 | ||||
Notes: Data come from the California Health Interview Survey (2009) and were weighted to represent the state population. Coding: 0 = no adherence; 1 = partial adherence; 2= full adherence. FMH denotes self-reported family history of cancer.
p < .001
p < 0.01
p < 0.05
p < 0.10 (two-tailed test)
Up-to-date with colorectal cancer screening (for adults aged 50–75) defined as one of: fecal occult blood (FOBT) test within past year, or sigmoidoscopy in past 5 years and FOBT in past 2, or colonoscopy within past 10 years.
Scale 0–12, with 0 indicating no adherence and 12 indicating full adherence. t-test used to test for significant differences by family history.
Energy dense food & drink is combination of fast food (0= at least once past week; 1= none) and sugary beverage consumption (0=≥4.5 servings/week; 1=<4.5servings/week).
Up-to-date breast cancer screening defined as mammography within the past 2 years for women, aged 40–75.
Notes: Data come from the California Health Interview Survey (2009) and were weighted to represent the state population. Coding: 0 = no adherence; 1 = partial adherence; 2= full adherence. FMH denotes self-reported family history of cancer.
p < .001;
p < 0.01;
p < 0.05;
p < 0.10 (two-tailed test)
Up-to-date with colorectal cancer screening (for adults aged 50–75) defined as one of: fecal occult blood (FOBT) test within past year, or sigmoidoscopy in past 5 years and FOBT in past 2, or colonoscopy within past 10 years.
Scale 0–12, with 0 indicating no adherence and 12 indicating full adherence. t-test used to test for significant differences by family history.
Energy dense food & drink is combination of fast food (0= at least once past week; 1= none) and sugary beverage consumption (0=≥4.5 servings/week; 1=<4.5servings/week).
Up-to-date breast cancer screening defined as mammography within the past 2 years for women, aged 40–75.
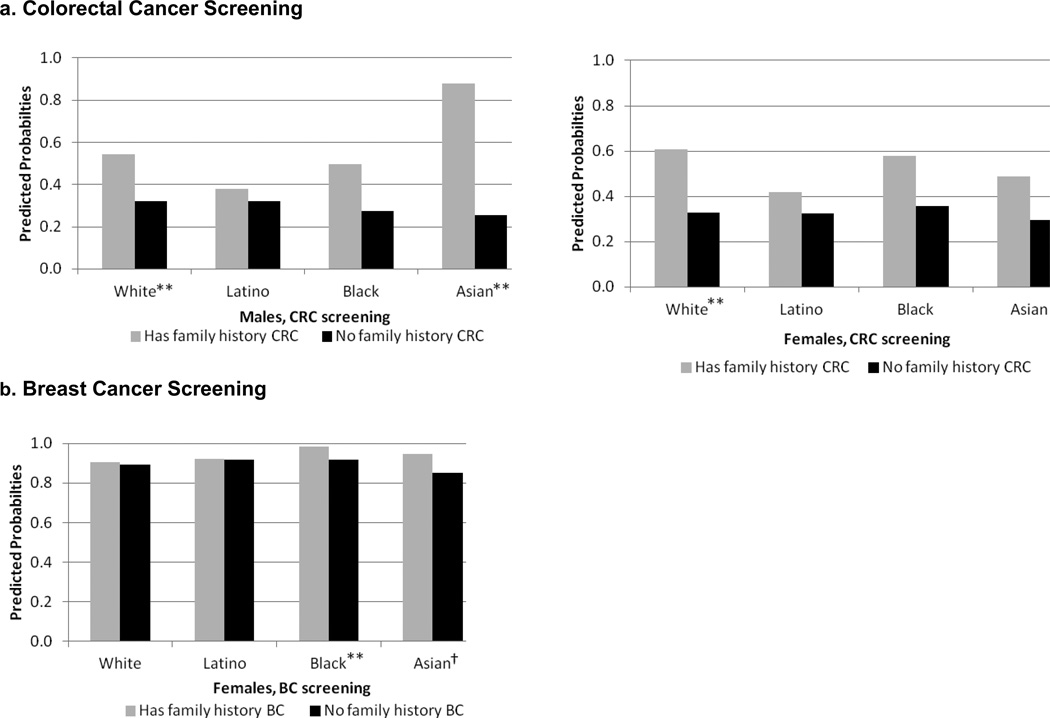
Figure 1. Predicted Probabilities of Cancer Screening by Family History of Site-Specific Cancer.
Source: Data come from the California Health Interview Survey (2009) and are weighted to be representative of the state population.
Notes: **p< .01, *p < .05, †p < .10 (two-tailed test of family history difference, Bonferroni-adjusted).
aPredicted probabilities holding the following variables constant at their mean values: age, marital status, education, income, current employment status, housing tenure, rural residence, doctor visit in past year, usual source of care, foreign-born status, English language proficiency
bUp-to-date with colorectal cancer screening (for adults aged 50–75) defined as one of: fecal occult blood (FOBT) test within past year, or sigmoidoscopy in past 5 years and FOBT in past 2, or colonoscopy within past 10 years.
cUp-to-date breast cancer screening defined as mammography within the past 2 years for women, aged 40–75.
dRespondent answered "yes" to the question, "Did your biological father or mother, full brothers or sisters, or biological sons or daughters ever have cancer of any kind?"
eRespondent reported having a first-degree blood relative who had colorectal or breast cancer, respectively.
Table 3.
Adjusteda Odds Ratios from Ordered Logistic or Poisson Regressions Predicting Greater Adherence to each Lifestyle/BMI Recommendation: Association with Family History
| Lifestyle scaleb |
Smoking | Physical Activity |
Energy dense food/drink |
Fruits & vegetables |
Alcohol | BMI | |
|---|---|---|---|---|---|---|---|
| Has family history (Reference: no family history) | |||||||
| Males | |||||||
| White | 0.97** | 0.88 | 0.81** | 1.01 | 0.973 | 1.050 | 0.82*** |
| (0.01) | (0.07) | (0.07) | (0.08) | (0.08) | (0.09) | (0.06) | |
| Latino | 0.98 | 0.88 | 0.57*** | 0.99 | 1.10 | 1.22 | 1.22 |
| (0.04) | (0.15) | (0.11) | (0.17) | (0.22) | (0.21) | (0.19) | |
| Black | 0.96 | 1.05 | 0.70 | 1.51 | 0.56* | 0.53* | 1.35 |
| (0.05) | (0.57) | (0.29) | (0.52) | (0.19) | (0.18) | (0.46) | |
| Asian | 0.95* | 0.95 | 1.42 | 0.77 | 0.76 | 0.49*** | 0.96 |
| (0.03) | (0.29) | (0.33) | (0.17) | (0.20) | (0.13) | (0.24) | |
| Females | |||||||
| White | 0.99 | 0.81*** | 1.04 | 0.87** | 1.10 | 1.07 | 0.94 |
| (0.01) | (0.06) | (0.07) | (0.06) | (0.07) | (0.07) | (0.07) | |
| Latino | 1.00 | 0.92 | 1.06 | 1.07 | 1.14 | 1.01 | 0.84 |
| (0.02) | (0.16) | (0.14) | (0.12) | (0.13) | (0.13) | (0.11) | |
| Black | 0.98 | 0.90 | 1.28 | 0.83 | 0.93 | 1.06 | 1.05 |
| (0.04) | (0.21) | (0.34) | (0.18) | (0.17) | (0.24) | (0.22) | |
| Asian | 0.99 | 0.50** | 0.91 | 0.81 | 1.26 | 0.82 | 1.34 |
| (0.02) | (0.14) | (0.22) | (0.17) | (0.21) | (0.15) | (0.28) | |
Notes: Data come from the California Health Interview Survey (2009) and were weighted to represent the state population. Standard errors in parentheses.
p < .001;
p < 0.01;
p < 0.05;
p < 0.10 (two-tailed test).
Regressions are separate by sex and race-ethnicity.
All dependent variables except Lifestyle Scale are coded: 0= no adherence; 1= partial adherence; 2=full adherence.
Adjusted for: age, marital status, education, income, current employment status, housing tenure, rural residence, doctor visit in past year, usual source of care, foreign-born status, English language proficiency.
Lifestyle Scale range (0–12); Poisson regression for count outcome.
Table 4.
Adjusteda Odds Ratios from Binomial Logistic Regressions Predicting Cancer Screening: Association with Adherence to Each Lifestyle/BMI Recommendation
| Males | ||||
|---|---|---|---|---|
| Colorectal Cancer Screening Up-to-dateb | ||||
| White | Latino | Black | Asian | |
| Lifestyle Adherence Scale (one point increase) | 1.07** | 1.09 | 0.96 | 1.13* |
| (0.03) | (0.06) | (0.10) | (0.07) | |
| Smoking (No adherence, Current Smoker, ref.) | ||||
| Partial adherence (Former Smoker) | 2.10*** | 1.17 | 2.95* | 2.38** |
| (0.43) | (0.44) | (1.71) | (0.95) | |
| Full adherence (Never Smoked) | 1.58** | 1.05 | 1.39 | 1.64 |
| (0.32) | (0.36) | (0.72) | (0.56) | |
| p-value from Wald test (full vs. partial) | 0.014 | 0.686 | 0.104 | 0.206 |
| Physical Activity (No adherence, <150 min./week, ref.)c | ||||
| Partial adherence (150–300 min./week) | 1.10 | 2.28** | 1.52 | 0.90 |
| (0.23) | (0.82) | (1.16) | (0.35) | |
| Full adherence (300+ min./week) | 1.11 | 1.28 | 0.97 | 1.89** |
| (0.15) | (0.36) | (0.53) | (0.55) | |
| p-value from Wald test (full vs. partial) | 0.97 | 0.15 | 0.60 | 0.09 |
| Foods that promote weight gain (No adherence, ref.) | ||||
| 1 (Partial adherence) | 1.17 | 1.49 | 0.50* | 0.90 |
| (0.19) | (0.41) | (0.18) | (0.30) | |
| 2 (Full adherence) | 1.14 | 1.51 | 0.55 | 1.34 |
| (0.21) | (0.46) | (0.35) | (0.50) | |
| p-value from Wald test (full vs. partial) | 0.82 | 0.96 | 0.86 | 0.18 |
| Fruits & vegetables (no adherence, 0–1.9 servings/day) | ||||
| Partial adherence (2–4.9 servings) | 1.47*** | 1.03 | 1.71 | 1.06 |
| (0.18) | (0.30) | (0.74) | (0.31) | |
| Full adherence (≥5 servings/day) | 1.43 | 0.97 | 0.39 | 0.86 |
| (0.32) | (0.85) | (0.60) | (0.78) | |
| p-value from Wald test (full vs. partial) | 0.29 | 0.88 | 0.95 | 0.32 |
| Alcohol consumption (No adherence, binge, ref.) | ||||
| Partial adherence (Some alcohol, no binge) | 1.07 | 1.26 | 0.39 | 1.61 |
| (0.14) | (0.35) | (0.23) | (0.68) | |
| Full adherence (None) | 0.99 | 1.06 | 0.80 | 1.58 |
| (0.15) | (0.32) | (0.48) | (0.72) | |
| p-value from Wald test (full vs. partial) | 0.81 | 0.64 | 0.58 | 0.11 |
| Body mass index (No adherence, <18.5 or >29.9 kg/m2, ref.) | ||||
| Partial adherence (25–29.9 kg/m2) | 1.11 | 1.31 | 1.83 | 0.61 |
| (0.14) | (0.36) | (0.74) | (0.24) | |
| Full adherence (18.5–24.9 kg/m2) | 1.07 | 1.44 | 0.77 | 0.84 |
| (0.14) | (0.48) | (0.33) | (0.33) | |
| p-value from Wald test (full vs. partial) | 0.94 | 0.78 | 0.78 | 0.04 |
| Females | ||||||||
|---|---|---|---|---|---|---|---|---|
| CRC Screening Up-to-dateb | BC Screening Up-to-dated | |||||||
| White | Latino | Black | Asian | White | Latino | Black | Asian | |
| Lifestyle Adherence Scale (one pt increase) | 1.03 | 1.01 | 0.98 | 0.89* | 1.04 | 1.04 | 1.05 | 0.99 |
| (0.02) | (0.04) | (0.08) | (0.06) | (0.03) | (0.07) | (0.10) | (0.09) | |
| Smoking (No adherence, Current Smoker, ref.) | ||||||||
| Partial adherence (Former Smoker) | 1.57** | 1.02 | 0.78 | 1.06 | 0.96 | 0.61 | 0.88 | 1.29 |
| (0.27) | (0.46) | (0.34) | (0.59) | (0.21) | (0.27) | (0.42) | (2.59) | |
| Full adherence (Never Smoked) | 1.55*** | 0.95 | 0.93 | 0.68 | 1.17 | 0.80 | 2.22* | 1.67 |
| (0.24) | (0.38) | (0.40) | (0.30) | (0.21) | (0.32) | (0.94) | (3.22) | |
| p-value from Wald test (full vs. partial) | 0.815 | 0.803 | 0.531 | 0.310 | 0.127 | 0.320 | 0.055 | 0.638 |
| Physical Activity (No adherence, <150 min./week, ref.)c | ||||||||
| Partial adherence (150–300 min./week) | 0.96 | 0.95 | 0.62 | 1.17 | 1.23 | 1.05 | 2.76 | 1.74 |
| (0.09) | (0.24) | (0.75) | (0.45) | (0.21) | (0.39) | (2.31) | (1.09) | |
| Full adherence (300+ min./week) | 0.99 | 1.49* | 2.88*** | 1.11 | 1.13 | 1.19 | 1.18 | 1.97 |
| (0.09) | (0.34) | (0.92) | (0.41) | (0.20) | (0.45) | (0.72) | (1.46) | |
| p-value from Wald test (full vs. partial) | 0.849 | 0.161 | 0.230 | 0.929 | 0.728 | 0.722 | 0.409 | 0.893 |
| Foods that promote weight gain (No adherence, ref.) | ||||||||
| 1 (Partial adherence) | 1.05 | 1.45 | 1.11 | 0.78 | 1.00 | 0.86 | 1.02 | 0.97 |
| (0.11) | (0.34) | (0.37) | (0.28) | (0.19) | (0.21) | (0.47) | (0.39) | |
| 2 (Full adherence) | 0.99 | 1.23 | 1.34 | 1.02 | 0.79 | 0.85 | 0.89 | 0.36** |
| (0.10) | (0.28) | (0.70) | (0.33) | (0.16) | (0.22) | (0.53) | (0.14) | |
| p-value from Wald test (full vs. partial) | 0.507 | 0.426 | 0.760 | 0.373 | 0.031 | 0.971 | 0.798 | 0.003 |
| Fruits & vegetables (no adherence, 0–1.9 servings/day) | ||||||||
| Partial adherence (2–4.9 servings) | 1.16* | 1.01 | 1.00 | 0.64 | 1.12 | 1.39 | 1.25 | 0.76 |
| (0.10) | (0.20) | (0.29) | (0.18) | (0.13) | (0.30) | (0.48) | (0.29) | |
| Full adherence (≥5 servings/day) | 1.03 | 0.82 | 1.26 | 0.42** | 0.98 | 0.72 | 1.83 | 0.62 |
| (0.13) | (0.25) | (0.62) | (0.18) | (0.19) | (0.26) | (1.70) | (0.39) | |
| p-value from Wald test (full vs. partial) | 0.226 | 0.501 | 0.667 | 0.371 | 0.513 | 0.050 | 0.686 | 0.691 |
| Alcohol consumption (No adherence, binge, ref.) | ||||||||
| Partial adherence (Some, no binge) | 1.06 | 0.95 | 0.57 | 1.16 | 1.39** | 1.17 | 0.21** | 3.49 |
| (0.11) | (0.24) | (0.24) | (0.54) | (0.20) | (0.42) | (0.15) | (2.98) | |
| Full adherence (None) | 1.05 | 1.05 | 0.41* | 1.25 | 1.07 | 1.51 | 0.30* | 2.29 |
| (0.13) | (0.24) | (0.19) | (0.58) | (0.18) | (0.46) | (0.19) | (1.90) | |
| p-value from Wald test (full vs. partial) | 0.966 | 0.595 | 0.363 | 0.800 | 0.091 | 0.333 | 0.397 | 0.127 |
| Body mass index (No adherence, <18.5 or >29.9 kg/m2, ref.) | ||||||||
| Partial adherence (25–29.9 kg/m2) | 1.20* | 0.94 | 1.03 | 1.53 | 1.03 | 1.16 | 1.56 | 2.37* |
| (0.12) | (0.21) | (0.46) | (0.69) | (0.16) | (0.30) | (0.79) | (1.12) | |
| Full adherence (18.5–24.9 kg/m2) | 1.06 | 0.83 | 0.59 | 0.62 | 1.30* | 1.12 | 0.53 | 1.84 |
| (0.10) | (0.18) | (0.21) | (0.24) | (0.18) | (0.34) | (0.24) | (0.76) | |
| p-value from Wald test (full vs. partial) | 0.167 | 0.538 | 0.252 | 0.003 | 0.096 | 0.889 | 0.052 | 0.496 |
Notes: Data come from the California Health Interview Survey (2009) and were weighted to represent the state population. Regressions are separate by sex and race-ethnicity. Bold indicates significant trend at .05 alpha level. Standard errors in parentheses.
p < .001;
p < 0.01;
p < 0.05;
p < 0.10 (two-tailed test).
Adjusted for: age, marital status, education, income, current employment status, housing tenure, rural residence, doctor visit in past year, usual source of care, foreign-born status, English language proficiency.
Up-to-date with colorectal cancer screening (for adults aged 50–75) defined as one of: fecal occult blood (FOBT) test within past year, or sigmoidoscopy in past 5 years and FOBT in past 2, or colonoscopy within past 10 years.
Physical Activity defined as moderate-equivalent minutes of leisure time activity in past week.
Up-to-date breast cancer screening defined as mammography within the past 2 years for women, aged 40–75.
Notes: Data come from the California Health Interview Survey (2009) and were weighted to represent the state population. Regressions are separate by sex and race-ethnicity. Bold indicates significant trend at .05 alpha level. Standard errors in parentheses.
p < .001;
p < 0.01;
p < 0.05;
p < 0.10 (two-tailed test).
Adjusted for: age, marital status, education, income, current employment status, housing tenure, rural residence, doctor visit in past year, usual source of care, foreign-born status, English language proficiency.
Up-to-date with colorectal cancer screening (for adults aged 50–75) defined as one of: fecal occult blood (FOBT) test within past year, or sigmoidoscopy in past 5 years and FOBT in past 2, or colonoscopy within past 10 years.
Physical Activity defined as moderate-equivalent minutes of leisure time activity in past week.
Up-to-date breast cancer screening defined as mammography within the past 2 years for women, aged 40–75.
Results
There were sociodemographic differences by family history among race-ethnic groups, for both sexes (Table 1). Those reporting a family history of cancer were generally older and had higher socioeconomic indicators (i.e., lower poverty rates and higher education). Latino males and White females with family history were more likely to have a usual source of care, and to have seen a doctor in the past 12 months. Those with family history were less likely to be foreign-born or have limited English proficiency.
Table 2 presents prevalence rates of screening and lifestyle behaviors by family history of cancer, stratified by sex and race-ethnicity. In terms of screening, among males, those with family history had higher rates of CRC screening than those with no family history, but the difference was only statistically significant for Asians (67.8% versus 53.2%). White males with a family history had the highest screening rates (68.8%), followed closely by Asian males with a family history (67.8%); Latinos and Blacks with a family history lagged behind (54.4% and 53.2%, respectively). Of males, Latinos with no family history had the lowest screening rates (45.6%). Among females, Asians and Whites with a family history had slightly higher CRC screening rates than their counterparts; the difference was only significant among Whites (69.2% versus 62.6%). White females with a family history had the highest screening rates, and Latino and Asian females with no family history had the lowest rates. BC screening was high in all groups, and the family history gap was marginally significant among Whites (91% versus 87.1%).
In terms of lifestyle behaviors, there were few significant differences by family history among both sexes (Table 2). There were no differences in overall adherence to lifestyle behaviors by family history for either males or females. Moreover, the direction of the association between family history and individual lifestyle behaviors varied. Males with a family history had higher rates of former smoking, but significantly higher fruit/vegetable consumption. There were marginally statistically significant differences in physical activity and BMI by family history, with lower lifestyle adherence among White males with family history. Among Latino males, those with a family history had lower rates of full lifestyle adherence, and higher partial adherence, to recommendations regarding foods and drinks that promote weight gain. Asian males with a family history were more likely to be non-adherent to recommended lifestyle behaviors compared to their counterparts with no family history. For all groups, rates of never smoking were lower, and former smoking rates higher, among those with a family history; rates of current smoking, on the other hand, were similar between family history groups. Of White females, fruit/vegetable consumption was higher, and rates of alcohol bingeing lower, among those with family history. Among Latinas, those with family history had lower rates of binge drinking, but were less likely to avoid alcohol completely (41.4% versus 48.4%) compared to those without a family history of cancer.
To address whether those with a family history of cancer are more likely to be up-to-date with cancer screening even accounting for sociodemographic differences, we conducted logistic regressions. Figure 1 presents predicted probabilities of cancer screening by family history of cancer, race-ethnicity and sex, calculated holding all covariates constant at their means. Individuals with a family history tended to have higher probabilities of screening, especially for CRC. Family history of CRC was associated with a significantly higher probability of CRC screening among Asian and White males, and White females. The probabilities of BC screening for all groups were much higher (around 80–90%) compared to CRC screening. Differences in BC screening rates by family history were small. Black and Asian women with family history of BC had higher probabilities of mammography than their race-ethnic counterparts with no family history.
Addressing our second research question, we found that, in contrast to the generally positive association between family history and cancer screening, few associations between family history and lifestyle behaviors were significant (Table 3). Family history was related to lower odds of adherence to recommendations in all cases where there were significant effects. For instance, compared to Latino males with no family history, those with a family history had almost half the odds of full adherence to physical activity recommendations (versus partial or no adherence) (OR= 0.57, p < .001). Black males with a family history had nearly half the odds of adherence to fruit/vegetable consumption and alcohol avoidance recommendations compared to their co-ethnics with no family history. Among Asians, males with a family history were nearly half as likely to adhere to recommendations to avoid alcohol, and females with a family history adhered less to recommendations to avoid smoking.
Speaking to our final research question, Table 4 presents the results of multivariate logistic regression analyses examining the association between cancer screening and lifestyle behaviors. In general, there were positive associations between lifestyle behaviors and cancer screening, with higher odds of screening among those with partial or full adherence to cancer prevention recommendations. This was particularly true for smoking, physical activity, and, among Whites, consumption of weight gain-promoting foods, and fruits and vegetables. Among White and Asian males, a one-point increase on the lifestyle adherence scale was associated with increased odds of CRC screening (6.5%–13.2%). For many lifestyle behaviors, there were few significant associations with screening, and the direction of the association varied by race-ethnicity. Tests examining differences between the odds ratios for partial and full adherence revealed few significant trends. Among females, overall lifestyle adherence was inversely associated only with CRC screening among Asians, with approximately 12% lower odds of screening for every one-point increase in adherence to cancer preventive lifestyle recommendations. Abstinence from smoking and more physical activity emerged as positively associated with screening for some groups, while full or partial adherence to guidelines on foods promoting weight gain, fruit and vegetables, and alcohol consumption emerged as negatively associated with screening for Blacks and/or Asians. We also examined whether the relationship between lifestyle behaviors and screening varied by family cancer history but found no significant interactions.
Discussion
Results addressed the three study research questions, showing that: 1. Family history of cancer was related to higher cancer screening (mainly CRC screening) for some groups; 2. Family history was largely unrelated to other cancer preventive behaviors/BMI, but in the few instances when family history was significantly related to lifestyle, individuals with a family history were less likely to adhere to recommendations; and, 3. Healthier lifestyle behaviors were associated with a higher likelihood of cancer screening for only some groups.
Rates of up-to-date cancer screening were higher among individuals with a family history of cancer, although the differences were statistically significant only for certain groups. Since we controlled for age, these effects cannot be attributed entirely to commencement of screening at a younger age in individuals with a family history. Plausible explanations are that individuals who know they have a family history of cancer may have greater perceived risk of cancer or be more likely to receive or adhere to a doctor’s recommendation for screening. The different relationship between family history and CRC versus BC screening may be due to differences in screening prevalence. BC screening prevalence is high in California, at approximately 90% compared to 72% nationally [23], with few disparities by race-ethnicity; therefore, there may be a ceiling effect for BC screening. By contrast, CRC screening rates are lower than for BC (approximately 65% nationally [24] and between 60%–63.5% in California [25]) and race-ethnic differences more pronounced. Differential barriers to screening may also explain greater disparities in CRC than BC. Meissner and colleagues [26] recently found that physicians report that patients' inability to pay is the main barrier for both BC and CRC screening. An additional barrier for CRC is that patients do not perceive it as a threat.
A major finding of this study is that family history predicts cancer screening, but is not associated with higher rates of other cancer preventive behaviors. Where family history was significantly related to lifestyle or BMI, individuals with a family history of cancer were less likely to adhere to lifestyle recommendations. Results from several recent studies are consistent with this finding [11, 12, 27]. There are several plausible explanations. First, it may reflect a process of intergenerational transmission of lifestyle behaviors whereby adult children who report a family history of cancer share similar behavioral risks as their family members. Previous research finds evidence of intergenerational transmission of health-risk behaviors between parents and their children [28]. For instance, those with a family history of cancer may be more likely to smoke because their parents smoked (and hence, have a cancer history). Second, the facilitators and barriers may differ for screening versus other cancer preventive behaviors. For example, individuals’ perceptions about effectiveness of lifestyle behaviors versus screening for cancer risk reduction may differ. It may be that most persons, even those with a family history, are not fully aware of the importance of health behaviors such as physical activity and diet for preventing cancer. A recent study supports this explanation; sisters of breast cancer patients perceived screening to be more effective in reducing breast cancer risk than physical activity or fruit/vegetable consumption, and perceived effectiveness was positively associated with engaging in these behaviors [29]. Nevertheless, if family history of cancer has been successfully used to increase cancer screening, whether through physician recommendations or patient education, the increased cancer screening alone may be enough to reduce cancer risk in this higher-risk population.
Lastly, healthier behaviors were generally associated with a higher likelihood of cancer screening, and this relationship did not vary by family cancer history. Therefore, while various cancer preventive behaviors may be correlated, there is not a stronger correlation or better behaviors among those with a family history. Among Blacks and Asians, adherence to some lifestyle behaviors was associated with lower odds of cancer screening.
Although we adjusted for education, income and other sociodemographic characteristics in our multivariate models, residual confounding may account for some of these differences by family history. In this study, those who reported family history of cancer were generally more likely to be college educated and have health insurance. Speaking to differences between those who report a family history and those who do not, the Structural Influence Model of Communication Inequality [30] suggests that social determinants such as education and income influence interpersonal health communication, including the discussion of family cancer history with family members—which ultimately affects health outcomes including knowledge, beliefs, health behaviors and screening [19]. Therefore, differences between individuals who report a family history of cancer and those who do not, may reflect differences in health communication or health literacy rather than actual family cancer history. Moreover, individuals with low levels of education may not associate their family history of colon cancer with increased vulnerability in the way that individuals with high levels of education appear to [31]. Thus, it is important to understand how self-reported family cancer history is associated with health literacy as well as cancer screening and behaviors.
Theories of health behavior may aid in the interpretation of these findings. The Health Belief Model [32], a cognitive, behavioral theory premised on rational decision-making by people with known risk of disease, has been shown to work well in predicting discrete, one-time health behaviors such as screening for bowel cancer [33]; however, the Health Belief Model is less useful in predicting lifestyle patterns which involve repeated choices such as diet [34]. For longer-term lifestyle behavior change, the Social Ecological Model, which focuses on social and environmental factors, may better identify consistent correlates of behavior [35]. As Gorin and colleagues have argued, more research is needed to understand repeated decision making, such as maintaining a cancer prevention behavior over time [36]. However, taken together, these theories support the notion that social structural factors, such as policies that promote cancer prevention activities, may be more important in shaping longer-term lifestyle behaviors than individual health education alone.
This study has limitations. The data were self-reported and cross-sectional. Although CHIS uses a probability sample, there may be selection bias. Moreover, while these results represent California, they may not be generalizable to the national population. Future research may address geographic variability in these findings, and the heterogeneity within the broad race-ethnic groups examined here. Future studies may also examine adherence to other recommendations not addressed here due to data constraints (e.g., American Cancer Society alcohol recommendations differ from those of the World Cancer Research Fund). Despite these limitations, this study has novel contributions: using a population-based sample to examine family history, investigating associations within race-ethnic groups and genders, and presenting predicted probabilities of cancer screening by race-ethnicity.
This study provides important insight into the role of self-reported family history of cancer in shaping two types of cancer prevention behaviors—screening and lifestyle—and how these are associated with one another. Given the mounting evidence that certain cancers are largely preventable, this study’s findings increase our understanding of cancer prevention behaviors, and how knowledge of one’s family history is related to such behaviors. Some scholars [5, 13] posit that family history might be used as a public health tool to identify those at higher risk of chronic diseases in order to tailor recommendations to individuals. Our findings suggest that knowledge of family history of cancer alone is insufficient to make individuals engage in cancer preventive lifestyle behaviors, but may be useful in increasing cancer screening. Interventions aimed at increasing awareness of the importance of health behaviors in cancer prevention, especially in this higher-risk group who have a family history of cancer, are needed. More research is needed to better understand individual decision-making about cancer prevention behaviors and the social structural and psychological factors influencing those behaviors.
Supplementary Material
Acknowledgments
During the preparation of this manuscript, the authors were supported by: R25CA087949 (Bostean; PI: Roshan Bastani); NIH CA16042 (Crespi); P50HL105188 (McCarthy).
Footnotes
Georgiana Bostean has no financial disclosures.
Catherine M. Crespi has no financial disclosures.
William J. McCarthy has no financial disclosures.
We use the WCRF-AICR recommendation cut-off for full adherence of 300 moderate-equivalent minutes/week in analyses shown here, but also conducted sensitivity analyses using the American Cancer Society recommendation of at least 150 moderate-equivalent minutes/week. Some associations are lost when using the dichotomous ACS cut-off, thus we chose to use the more nuanced measure in final analyses.
References
- 1.Kushi LH, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA: A Cancer Journal for Clinicians. 2012;62(1):30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 2.World Cancer Research Fund International/American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: a global perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 3.Hemminki K, Sundquist J, Bermejo JL. How common is familial cancer? Annals of Oncology. 2008;19(1):163–167. doi: 10.1093/annonc/mdm414. [DOI] [PubMed] [Google Scholar]
- 4.Slattery ML, et al. Family history and colorectal cancer: predictors of risk. Cancer Causes and Control. 2003;14(9):879–887. doi: 10.1023/b:caco.0000003840.94591.76. [DOI] [PubMed] [Google Scholar]
- 5.Valdez R, et al. Family History in Public Health Practice: A Genomic Tool for Disease Prevention and Health Promotion. Annual Review of Public Health. 2010;31(1):69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 6.Bastani R, et al. Validation of Self-reported Colorectal Cancer (CRC) Screening in a Study of Ethnically Diverse First-Degree Relatives of CRC Cases. Cancer Epidemiology Biomarkers & Prevention. 2008;17(4):791–798. doi: 10.1158/1055-9965.EPI-07-2625. [DOI] [PubMed] [Google Scholar]
- 7.Bastani R, et al. Tailored Risk Notification for Women with a Family History of Breast Cancer. Preventive Medicine. 1999;29(5):355–364. doi: 10.1006/pmed.1999.0556. [DOI] [PubMed] [Google Scholar]
- 8.Glenn BA, et al. Changes in risk perceptions in relation to self-reported colorectal cancer screening among first-degree relatives of colorectal cancer cases enrolled in a randomized trial. Health Psychology. 2011;30(4):481–491. doi: 10.1037/a0024288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martínez-Ochoa E, et al. Influence of Family History of Colorectal Cancer on Health Behavior and Performance of Early Detection Procedures: The SUN Project. Annals of Epidemiology. 2012;22(7):511–519. doi: 10.1016/j.annepidem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Ponce NA, et al. Disparities in cancer screening in individuals with a family history of breast or colorectal cancer. Cancer. 2012;118(6):1656–1663. doi: 10.1002/cncr.26480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend JS, et al. Health behaviors and cancer screening among Californians with a family history of cancer. Genetics in Medicine. 2013;15(3):212–221. doi: 10.1038/gim.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madlensky L, et al. Preventive Health Behaviors and Familial Breast Cancer. Cancer Epidemiology Biomarkers & Prevention. 2005;14(10):2340–2345. doi: 10.1158/1055-9965.EPI-05-0254. [DOI] [PubMed] [Google Scholar]
- 13.Yoon PW, et al. Can family history be used as a tool for public health and preventive medicine? Genetics in Medicine. 2002;4(4):304. doi: 10.1097/00125817-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Claassen L, et al. Using family history information to promote healthy lifestyles and prevent diseases; a discussion of the evidence. BMC Public Health. 2010;10(1):248. doi: 10.1186/1471-2458-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrigan D, et al. Patterns of health behavior in U.S. adults. Preventive Medicine. 2003;36(5):615–623. doi: 10.1016/s0091-7435(02)00067-1. [DOI] [PubMed] [Google Scholar]
- 16.Kaczynski AT, et al. Smoking and Physical Activity: A Systematic Review. American Journal of Health Behavior. 2008;32(1):93–110. doi: 10.5555/ajhb.2008.32.1.93. [DOI] [PubMed] [Google Scholar]
- 17.California Health Interview Survey. Report 1 - Sample Design. In: UCLA Center for Health Policy Research, editor. CHIS 2009 Methodology Series. Los Angeles, CA: UCLA; 2011. [Google Scholar]
- 18.California Health Interview Survey. Report 4 – Response Rates. In: U.C.f.H.P. Research, editor. California Health Interview Survey 2009 Methodology Report Series. Los Angeles, CA: UCLA; 2011. [Google Scholar]
- 19.National Cancer Institute. [cited 8/9/2012];Health Information National Trends Survey 2003: Final Report. 2003 Oct. 2003:[Available from: http://hints.cancer.gov/docs/HINTS_2003_final_report.pdf.
- 20.US Preventive Services Task Force. [cited Aug. 9, 2012];Recommendations for Adults. 2012 Available from: http://www.uspreventiveservicestaskforce.org/adultrec.htm.
- 21.US Preventive Services Task Force. Screening for Breast Cancer. 2009 [cited; Available from: http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm.
- 22.StataCorp. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 23.Centers for Disease Control and Prevention. Cancer Screening — United States, 2010. Morbidity and Mortality Weekly Report. 2012;61(3) [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services. Percentage of Adults Who Receive Colorectal Cancer Screening as Appropriate: National Health Interview Survey. [cited 2013 3/26];2013 Available from: https://healthmeasures.aspe.hhs.gov/measure/25#additionalInformation.
- 25.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Data. [cited 3/26/2013];2010 11/27/2012 Available from: http://www.cdc.gov/cancer/colorectal/statistics/screening_rates.htm.
- 26.Meissner HI, et al. Breast and Colorectal Cancer Screening: U.S. Primary Care Physicians' Reports of Barriers. American Journal of Preventive Medicine. 2012;43(6):584–589. doi: 10.1016/j.amepre.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Spector D, DeRoo LA, Sandler DP. Lifestyle behaviors in Black and White women with a family history of breast cancer. Preventive Medicine. 2011;52(5):394–397. doi: 10.1016/j.ypmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickrama KAS, et al. The Intergenerational Transmission of Health-Risk Behaviors: Adolescent Lifestyles and Gender Moderating Effects. Journal of Health and Social Behavior. 1999;40(3):258–272. [PubMed] [Google Scholar]
- 29.Hartman SJ, Dunsiger SI, Jacobsen PB. The relationship of psychosocial factors to mammograms, physical activity, and fruit and vegetable consumption among sisters of breast cancer patients. International journal of women's health. 2011;3:257–263. doi: 10.2147/IJWH.S23246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viswanath K, Ramanadhan S, Kontos EZ. Mass Media. In: Galea S, editor. Macrosocial Determinants of Population Health. New York, NY: Springer; 2007. pp. 275–294. [Google Scholar]
- 31.Courtney RJ, et al. A population-based cross-sectional study of colorectal cancer screening practices of first-degree relatives of colorectal cancer patients. BMC Cancer C7 – 13. 2013;13(1):1–11. doi: 10.1186/1471-2407-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenstock IM. Historical Origins of the Health Belief Model. Health Education and Behavior. 1974;2(4):328–335. [Google Scholar]
- 33.Wardle J, et al. Psychosocial Influences on Older Adults' Interest in Participating in Bowel Cancer Screening. Preventive Medicine. 2000;31(4):323–334. doi: 10.1006/pmed.2000.0725. [DOI] [PubMed] [Google Scholar]
- 34.Carpenter CJ. A Meta-Analysis of the Effectiveness of Health Belief Model Variables in Predicting Behavior. Health Communication. 2010;25(8):661–669. doi: 10.1080/10410236.2010.521906. [DOI] [PubMed] [Google Scholar]
- 35.Bronfenbrenner Ur. Environments in Developmental Perspective: Theoretical and operational model. In: Friedman SL, Wachs TD, editors. Measuring environment across the life span: Emerging methods and concepts. Washington, DC: American Psychological Association Press; 1999. pp. 3–28. [Google Scholar]
- 36.Gorin S, et al. Decision making in cancer primary prevention and chemoprevention. Annals of Behavioral Medicine. 2006;32(3):179–187. doi: 10.1207/s15324796abm3203_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.