SUMMARY
Mouse hematopoiesis is initiated by long-term hematopoietic stem cells (HSC) that differentiate into a series of multipotent progenitors that exhibit progressively diminished self-renewal ability. In human hematopoiesis, populations enriched for HSC activity have been identified, as have downstream lineage-committed progenitors, but multipotent progenitor activity has not been uniquely isolated. Previous reports indicate that human HSC are enriched in Lin-CD34+CD38- cord blood and bone marrow and express CD90. We demonstrate that the Lin-CD34+CD38- fraction of cord blood and bone marrow can be subdivided into three subpopulations: CD90+CD45RA-, CD90-CD45RA-, and CD90-CD45RA+. Utilizing in vivo transplantation studies and complementary in vitro assays, we demonstrate that the Lin-CD34+CD38-CD90+CD45RA- cord blood fraction contains HSC, and isolate this activity to as few as 10 purified cells. Furthermore, we report the first prospective isolation of a population of candidate human multipotent progenitors, Lin-CD34+CD38-CD90-CD45RA- cord blood.
INTRODUCTION
Hematopoiesis proceeds through an organized developmental hierarchy initiated by hematopoietic stem cells (HSC) that give rise to progressively more committed progenitors and eventually terminally differentiated blood cells (Bryder et al., 2006). Although the concept of the HSC was not new, it was not until 1988 that it was shown that this population could be prospectively isolated from mouse bone marrow on the basis of cell-surface markers using fluorescence-activated cell sorting (FACS) (Spangrude et al., 1988). Since that time, the surface immunophenotype of the mouse HSC has become increasingly refined, such that functional HSC can be isolated with exquisite sensitivity, resulting in a purity of 1 in 1.3 cells (Kiel et al., 2005). While our ability to prospectively isolate mouse HSC has improved dramatically over the past 20 years, our understanding of the earliest events in the human hematopoietic system lags far behind.
Both in vitro and in vivo experimental approaches have been utilized to identify human HSC (Kondo et al., 2003). The best in vitro assay of HSC activity is the long-term culture-initiating cell assay (LTC-IC), which requires culturing of cells on bone marrow feeder cells to identify those capable of producing hematopoietic cells for 6 weeks or longer (Sutherland et al., 1989). Using this technique, candidate human HSC were identified by virtue of expression of CD34 and CD90 (Thy1) and lack of lineage markers (Lin-) (Baum et al., 1992; Craig et al., 1993). Other studies using the LTC-IC assay localized human HSC activity to the Lin-CD34+CD38-/lo fraction (Kondo et al., 2003). Although these in vitro assays provide important information regarding lineage potential and possibly self-renewal, it is clear that definitive demonstration of HSC function requires an in vivo assay.
Several in vivo models have been utilized to study human hematopoiesis. McCune and colleagues used the SCID-hu mouse model to identify human HSC activity among Lin-CD34+CD90+ cells (McCune et al., 1988; Murray et al., 1995; Peault et al., 1993). Dick and colleagues initially used SCID/beige/XID, and more recently NOD/SCID mice, to assay normal human progenitor subpopulations (Dick et al., 2001). By assessing long-term multipotent human hematopoiesis in recipients and the ability to form secondary and tertiary transplants, human HSC were found to reside in the Lin-CD34+CD38-/lo fraction of human progenitors (Bhatia et al., 1997; Cashman et al., 1997; Hogan et al., 2002). Recently, the NOD/SCID/IL-2Rγ-null strain (NOG) strain was shown to exhibit significantly higher engraftment potential than other immunodeficient mouse strains when transplanted with human hematopoietic progenitors (Shultz et al., 2005). Transplantation of 20,000 Lin-CD34+CD38- human cord blood cells into sublethally irradiated newborn pups resulted in multi-lineage engraftment, with significant numbers of human cells present in the peripheral blood (Ishikawa et al., 2005). Perhaps the best in vivo demonstration of HSC function comes from human trials of autologous mobilized peripheral blood in clinical transplantation, where long-term engraftment was provided by transplantation of purified CD34+CD90+ cells (Michallet et al., 2000; Negrin et al., 2000; Vose et al., 2001). Together, these in vivo studies support the idea that human HSC are contained in the Lin-CD34+CD38-CD90+ fraction of hematopoietic cells.
HSC are defined on the basis of two key properties: (1) multipotency, defined as the ability to form all differentiated blood cells, and (2) long-term self-renewal, defined as the lifelong ability to give rise to progeny identical to the parent through cell division. HSC are the only hematopoietic cells that possess these two properties; however, there are hematopoietic cells that are multipotent, but not capable of long-term self-renewal, termed multipotent progenitors (MPP). MPP lie immediately downstream of HSC within the hematopoietic hierarchy, and have been identified in mouse bone marrow on the basis of their distinct surface immunophenotype (Christensen and Weissman, 2001; Morrison et al., 1997; Morrison and Weissman, 1994). MPP have yet to be identified within the human hematopoiesis hierarchy, but functional evidence suggests that they exist. Retroviral or lentiviral marking of Lin-CD34+CD38-/lo cells prior to xenotransplantation allowed investigators to track engrafted cells, resulting in the observation that individual Lin-CD34+CD38-/lo cells had variable self-renewal and proliferation potential (Guenechea et al., 2001; Mazurier et al., 2004; McKenzie et al., 2006). Although it was not determined if the short-term clones were multipotent, these observations suggest that MPP are contained within the Lin-CD34+CD38-/lo fraction of human hematopoietic progenitors.
In this report, we demonstrate that the Lin-CD34+CD38- fraction of human cord blood and bone marrow can be divided into three subpopulations based on expression of CD90 and CD45RA: CD90+CD45RA-, CD90-CD45RA-, and CD90-CD45RA+. Using in vivo xenotransplantation as well as complementary in vitro assays, we show that the Lin-CD34+CD38-CD90+CD45RA- cord blood population contains human HSC, and isolate this activity to as few as 10 purified cells. In addition, we demonstrate that this population resides at the top of the human hematopoietic developmental hierarchy. Finally, we report the first identification and prospective isolation of a population of candidate human multipotent progenitors, Lin-CD34+CD38-CD90-CD45RA- cord blood.
RESULTS
Identification of CD90/CD45RA Subpopulations of Lin-CD34+CD38- Human Bone Marrow and Cord Blood
Data from multiple investigators indicate that human HSC activity resides in the CD90+ fraction of Lin-CD34+CD38-/lo cells. Using the marker CD45RA, three subpopulations of Lin-CD34+CD38- bone marrow and cord blood were identified: (1) CD90+CD45RA-, (2) CD90-CD45RA-, and (3) CD90-CD45RA+ (Figure 1). In the bone marrow these population comprised 30.3 ± 18.9% (CD90+CD45RA-), 37.7 ± 14.1% (CD90-CD45RA-), and 24.7 ± 11.8% (CD90-CD45RA) of Lin-CD34+CD38- cells (n=10). In cord blood, these fractions constituted 25.2 ± 10.3% (CD90+CD45RA-), 49.8 ± 11.4% (CD90-CD45RA-), and 18.4 ± 8.4% (CD90-CD45RA+) of Lin-CD34+CD38-cells (n=22). All three subpopulations were isolated to > 95% purity from cord blood and bone marrow by FACS (Supplementary Figure 1).
Figure 1. Identification of CD90/CD45RA Subpopulations of Lin-CD34+CD38-Human Bone Marrow and Cord Blood.
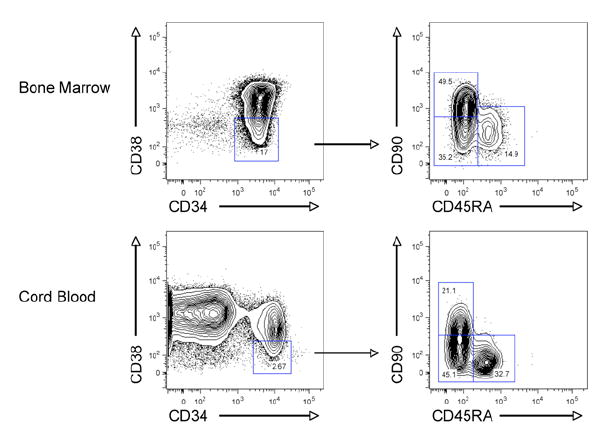
Normal human bone marrow (top) and cord blood (bottom) were analyzed for expression of lineage markers, CD34, CD38, CD90, and CD45RA by flow cytometry. The bone marrow sample was CD34-enriched prior to analysis. The left panels are gated on lineage negative (Lin-) live cells, while the right panels are gated on Lin-CD34+CD38- cells. Data shown is representative of multiple samples of bone marrow (n=10) and cord blood (n=22).
Methylcellulose Colony Formation and Replating of CD90/CD45RA Subpopulations
In order to assess the lineage potential of the CD90/CD45RA subpopulations, each was assayed for in vitro colony formation in methylcellulose. Single cells of each population were sorted into individual wells of a 96-well plate containing complete methylcellulose. In all cases, only a single colony was detected. CD90+CD45RA- cells formed all types of myeloid colonies, as did CD90-CD45RA- cells (Figure 2A). No differences were detected in plating efficiency or colony subtype distribution between these two subpopulations; however, the CD90+CD45RA- colonies were generally much larger and faster growing. CD90-CD45RA+ cells formed very few colonies, suggesting that these cells possess limited myeloid differentiation potential.
Figure 2. In Vitro Evaluation of the CD90/CD45RA Subpopulations of Lin-CD34+CD38- Cord Blood Reveals a Developmental Hierarchy.
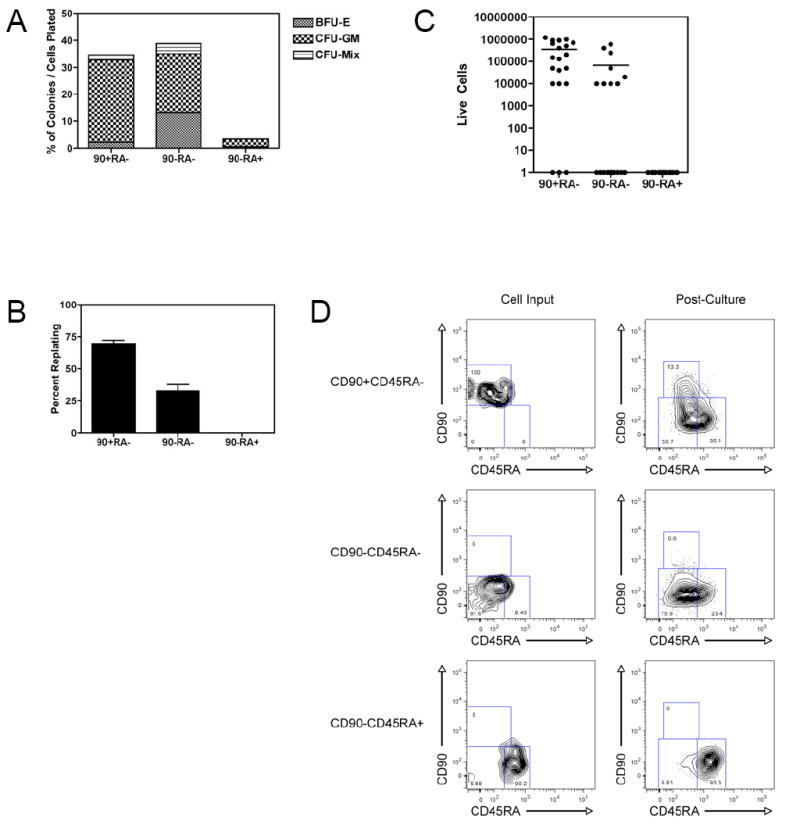
A. Methylcellulose colony formation
Single cells from each CD90/CD45RA subpopulation were sorted into individual wells of a 96 well plate containing complete methylcellulose capable of supporting growth of all types of myeloid colonies. After 12-14 days, colonies were scored based on morphology. The percent of each type of colony out of the total cells plated is indicated. Data presented is cumulative from 3 experiments of 60 cells each, for a total of 180 cells per subpopulation.
B. Methylcellulose colony replating
All colonies derived from individual cells were harvested, dissociated, and replated in complete methylcellulose. 12-14 days later, the formation of new colonies was scored based on morphology. 42 out of 62 (70%) of CD90+CD45RA-, 23 out of 70 (33%) of CD90-CD45RA-, and 0 out of 6 (0%) of CD90-CD45RA+ colonies formed new colonies upon replating. The difference in replating efficiency between CD90+CD45RA- and CD90-CD45RA- was statistically significant (p=0.003). Data presented is the average of 3 independent experiments with the indicated SEM.
C. In vitro proliferation
20 cells of each CD90/CD45RA subpopulation were clone sorted into individual wells of a 96 well plate containing serum-free media supplemented with human LDL and cytokines. After 2 weeks in culture, cells were harvested and live cells counted by trypan blue exclusion. The difference between the CD90+CD45RA- and the CD90-CD45RA- subpopulations was statistically significant (p=0.007). Data is representative of 3 independent experiments.
D. In vitro hierarchical relationships among CD90/CD45RA subpopulations
CD90/CD45RA subpopulations were sorted in bulk into serum-free media supplemented with human LDL and cytokines. Cells were cultured for four days and then re-analyzed by flow cytometry. All plots shown are gated on Lin-CD34+CD38- cells; the left panels show the cell input; the right panels show the cells after four days in culture. Data shown is representative of 3 independent experiments.
All colonies derived from individual cells were then harvested, dissociated, and plated in complete methylcellulose in order to determine replating efficiency, an in vitro surrogate for self-renewal (Figure 2B). 70% of colonies derived from CD90+CD45RA-cells were able to form new colonies (in most cases, hundreds) upon replating. 33% of colonies derived from CD90-CD45RA- cells were able to form new colonies (in most cases, fewer than 50). None of the colonies derived from CD90+CD45RA- cells were able to form new colonies; however, very few colonies formed in the first plating (n=6). Thus, the CD90-CD45RA- subpopulation is able to form all myeloid cells, but has reduced capacity for self-renewal compared to the CD90+CD45RA- subpopulation, which is presumed to contain HSC.
In Vitro Proliferation and Differentiation Identifies a Hierarchy Among the CD90/CD45RA Subpopulations
The CD90/CD45RA subpopulations were also assayed for in vitro proliferation in serum-free liquid culture. Single cells of each population were sorted into individual wells of a 96-well plate containing serum-free media supplemented with Flt-3 ligand, SCF, TPO, IL-3, and IL-6 and cultured for 2 weeks. At the end of the culture period, live cells were counted. CD90+CD45RA- cells proliferated extensively with a mean recovery of 345,000 cells; CD90-CD45RA- cells proliferated to a lesser degree with a mean recovery of 67,500 cells; CD90-CD45RA+ cells proliferated poorly with few live cells recovered (Figure 2C). These results support the observed differences in methylcellulose colony size and suggest that the CD90- subpopulations are less primitive than the CD90+ subpopulation presumed to contain HSC.
In order to examine the potential hierarchical relationships between the CD90/CD45RA subpopulations within the Lin-CD34+CD38- fraction, each population was sorted in bulk into serum-free media supplemented with cytokines as described above. After four days in culture, the cells were re-analyzed for expression of CD90 and CD45RA (Figure 2D). While CD90+CD45RA- cells gave rise to all three subpopulations, CD90-CD45RA- cells gave rise to both CD90- subpopulations, but not CD90+ cells. CD90-CD45RA+ cells gave rise principally to itself only. Together, these data establish an in vitro differentiation hierarchy in which CD90+CD45RA- cells give rise to CD90-CD45RA- cells, which in turn give rise to CD90-CD45RA+ cells.
Long-Term In Vivo Multipotent Human Hematopoiesis with Transplantation of as few as 10 Lin-CD34+CD38-CD90+CD45RA- Cord Blood Cells
Newborn NOD/SCID/IL-2Rγ-null (NOG) mice were used in xenotransplantation assays to determine the differentiation potential and self-renewal ability of the CD90/CD45RA subpopulations. Transplantation of 100 purified CD90+CD45RA- cells resulted in circulating human CD45+ hematopoietic cells at 12 weeks, including both CD13+ myeloid cells, and CD19+ B cells, but not CD3+ T cells (Figure 3A). Several of these mice were followed beyond 12 weeks (maximum 30 weeks), and all continued to have detectable human myeloid cells at similar levels in the peripheral blood (data not shown), indicating continued human engraftment. In order to isolate this activity to as few cells as possible, 10 purified CD90+CD45RA- cells were transplanted. At 12 weeks, few circulating human CD45+ cells were detected, and no CD13+ myeloid cells were present (Figure 3A); however, analysis of the bone marrow showed significant human engraftment with both myeloid and lymphoid cells (Figure 3A). Successful long-term human engraftment was observed in 3 out of 10 mice transplanted with 10 CD90+CD45RA- cells, with the 3 successful engraftments coming from independent cord blood samples.
Figure 3. Long-Term In Vivo Multipotent Human Hematopoiesis with Transplantation of as few as 10 Lin-CD34+CD38-CD90+CD45RA- Cord Blood Cells.
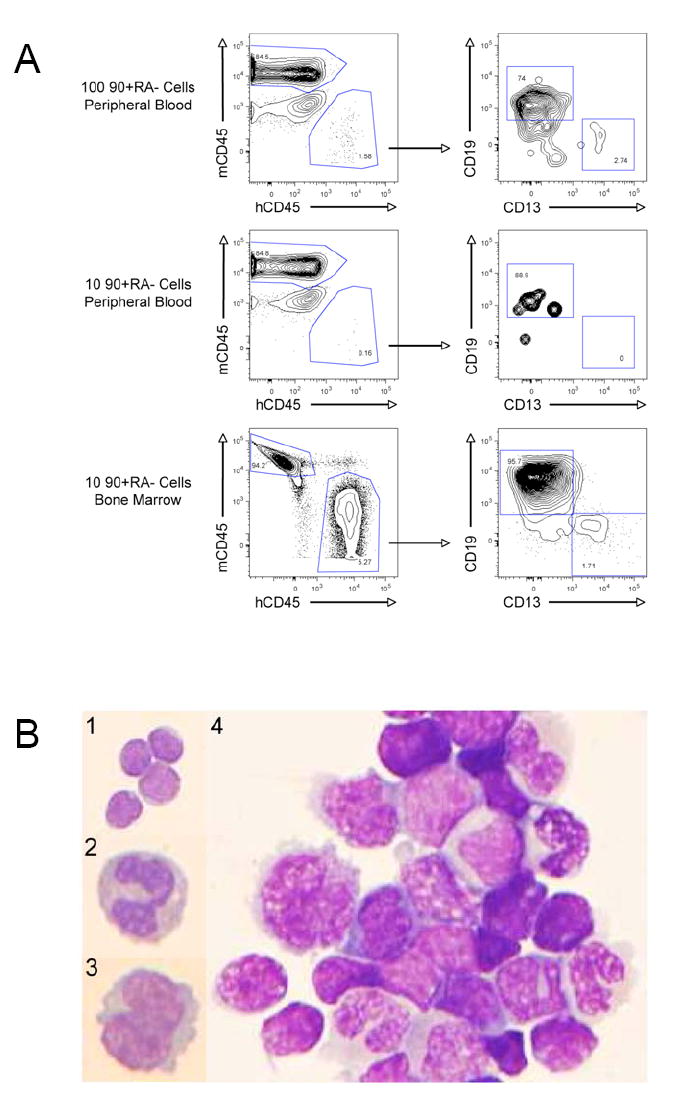
A. In vivo engraftment of 100 or 10 CD90+CD45RA- cells
100 or 10 FACS-purified CD90+CD45RA- cells were transplanted into NOG newborn mice as described. 12 weeks later peripheral blood and/or bone marrow was harvested and analyzed by flow cytometry for the presence of human CD45+ hematopoietic cells, myeloid cells (hCD45+CD13+), and B lymphoid cells (hCD45+CD19+) cells. The right plots are gated on human CD45+ cells.
B. Wright-Giemsa stained cytospin preparations from CD90+CD45RA- engrafted mice
Human CD45+ cells were purified by FACS from peripheral blood (panels 1-3) or bone marrow (panel 4) of mice engrafted with CD90+CD45RA- cells. In the peripheral blood, (1) lymphocytes, (2) neutrophils, and (3) monocytes were detected; in the bone marrow (4) lymphocytes and maturing myeloid cells were detected. (100x)
Both CD13+ myeloid cells and CD19+ B cells were detected in the blood and bone marrow of engrafted mice, indicating that CD90+CD45RA- cells possess lymphoid and myeloid potential, and are likely multipotent. Analysis of the spleen from engrafted mice identified human CD45+CD3+ T cells (Supplementary Figure 2), and staining of the bone marrow with glycophorin-A and CD61/41 identified human erythroid cells and platelets (data not shown). To confirm this flow cytometry-derived lineage analysis, human CD45+ cells from both peripheral blood and bone marrow were FACS-purified and cytospin preparations were stained with Wright-Giemsa. These stains confirmed the presence of mature human lymphocytes, neutrophils, and monocytes in the peripheral blood (Figure 3B, panels 1-3). In the bone marrow, both lymphocytes and maturing myeloid cells were readily detected (Figure 3B, panel 4). Collectively, these data indicate that Lin-CD34+CD38-CD90+CD45RA- cord blood cells are capable of establishing long-term in vivo multipotent human hematopoiesis and that this activity can be isolated to as few as 10 cells.
Long-Term In Vivo Multipotent Human Hematopoiesis Requires Transplantation of More CD90-CD45RA- Cells Than CD90+CD45RA- Cells
All three CD90/CD45RA subpopulations of cord blood were purified by FACS and transplanted into NOG mice. Transplantation of both the CD90+CD45RA- and the CD90-CD45RA- subpopulations resulted in detectable human myeloid and B lymphoid cells in the peripheral blood 12 weeks after transplantation (Figure 4A). No human cells were detected in the peripheral blood of mice transplanted with the CD90-CD45RA+ subpopulation, even at time points as early as 4 weeks after transplantation (Figure 4A). Multiple transplantation experiments were conducted with independent cord blood samples resulting in transplantation of between 100 and 4440 cells of each population (Figure 4B). The cumulative data from these experiments showed that 12 of 13 mice (92%) transplanted with CD90+CD45RA- cells, and 15 of 17 mice (88%) transplanted with CD90-CD45RA- cells contained human CD45+ cells in the peripheral blood at least 12 weeks after transplantation (Figure 4B). In the engrafted mice, the average human CD45+ chimerism was 3.7% for the CD90+CD45RA- transplants compared to 4.0% for the CD90-CD45RA- transplants; the percentage of myeloid cells among human CD45+ cells was 6.7% for the CD90+CD45RA- transplants compared to 7.1% for the CD90-CD45RA- transplants (Figure 4B). Because different cell numbers were used in each of these transplantation experiments, the engraftment per 100 transplanted cells was determined. The CD90+CD45RA- engrafted mice averaged 7 fold greater human chimerism and 7 fold greater human myeloid cells than the CD90-CD45RA- engrafted mice (Figure 4C). The difference in human chimerism was statistically significant with p=0.02, while the difference in human myeloid cells approached statistical significance with p=0.08.
Figure 4. Human Lymphoid and Myeloid Cells Reconstitute the Peripheral Blood of CD90/CD45RA Transplanted Mice.
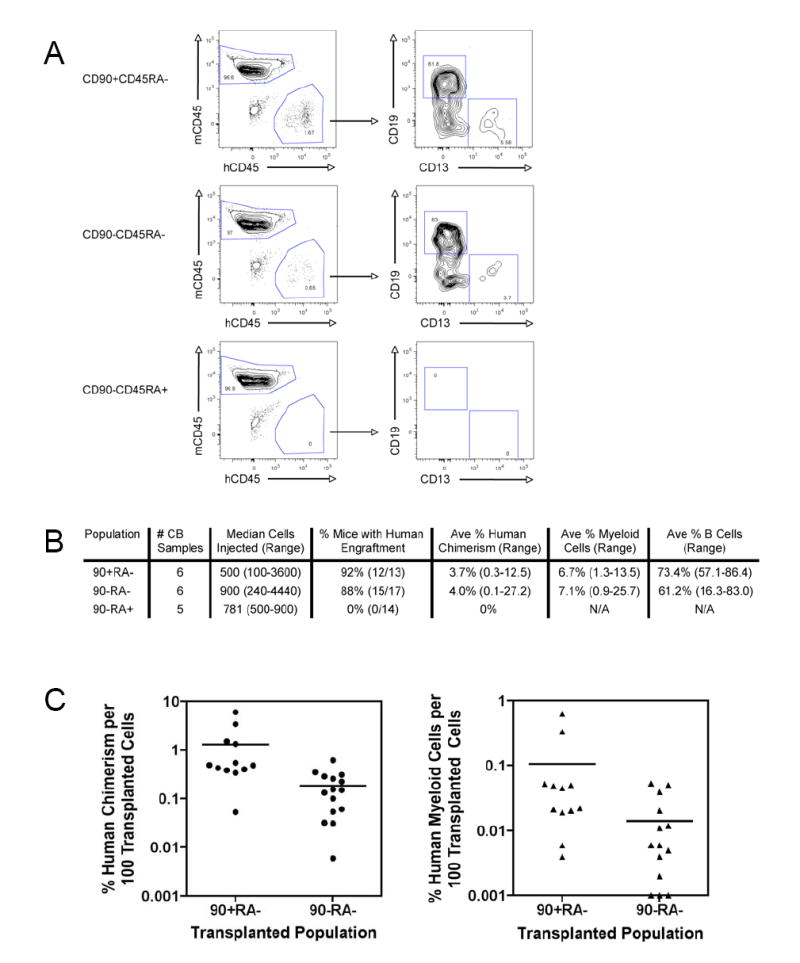
A. Peripheral blood engraftment and lineage analysis of CD90/CD45RA transplanted mice
500 FACS-purified cells of each CD90/CD45RA subpopulation: CD90+CD45RA- (top panels), CD90-CD45RA- (middle panels), and CD90-CD45RA+ (bottom panels), were transplanted into NOG newborn mice as described. 12 weeks later peripheral blood was harvested and analyzed by flow cytometry for the presence of human CD45+ hematopoietic cells, myeloid cells (hCD45+CD13+), and B lymphoid cells (hCD45+CD19+) cells. The right plots are gated on human CD45+ cells.
B. Summary of long-term (> 12 weeks) peripheral blood engraftment of CD90/CD45RA subpopulations
C. Peripheral blood engraftment per 100 transplanted cells
The percent human chimerism (left) and percent human myeloid cells (right) per 100 transplanted cells is indicated for each engrafted mouse. Each circle or triangle represents an individual mouse and the bar indicates the average. On average, CD90+CD45RA- mice developed 7 fold more human chimerism than CD90-CD45RA- mice, and this difference was statistically significant (p=0.02). The 7 fold difference in percent human myeloid cells approached, but did not achieve statistical significance (p=0.08).
Analysis of the bone marrow of transplanted mice revealed human myeloid and B cells 12 weeks after transplantation of CD90+CD45RA- and CD90-CD45RA- cells (Figure 5A). No human cells were detected in the bone marrow of mice transplanted with the CD90-CD45RA+ population (data not shown). Cumulative data showed that 8 of 8 mice (100%) transplanted with CD90+CD45RA- cells, and 8 of 9 mice (89%) transplanted with CD90-CD45RA- cells contained human CD45+ cells in the bone marrow at least 12 weeks after transplantation (Figure 5B). In the engrafted mice, the average human chimerism was 19.9% for the CD90+CD45RA- transplants compared to 4.4% for the CD90-CD45RA- transplants; the percent myeloid of total human CD45+ cells was 24.3% for the CD90+CD45RA- transplants compared to 26.6% for the CD90-CD45RA- transplants (Figure 5B). In order to normalize for cell number, bone marrow engraftment per 100 transplanted cells was determined. The CD90+CD45RA- engrafted mice averaged 9 fold greater human chimerism and 9 fold greater human myeloid cells than the CD90-CD45RA- engrafted mice (Figure 5C). The difference in human chimerism was statistically significant with p=0.001, while the difference in human myeloid cells approached statistical significance with p=0.07.
Figure 5. Human Lymphoid and Myeloid Cells Reconstitute the Bone Marrow of CD90+CD45RA- Transplanted Mice More Efficiently than CD90-CD45RA-Transplanted Mice.
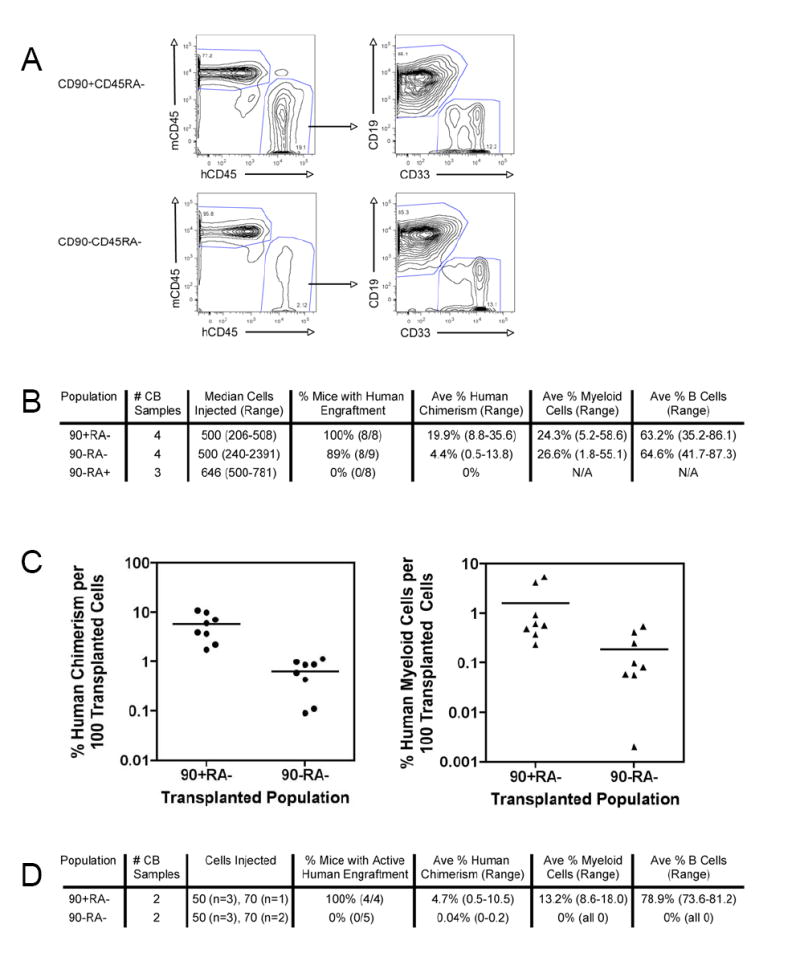
A. Bone marrow engraftment and lineage analysis of CD90/CD45RA transplanted mice
500 FACS-purified CD90+CD45RA- cells (top panels) and CD90-CD45RA- cells (bottom panels) were transplanted into NOG newborn mice as described. 12 weeks later bone marrow was analyzed by flow cytometry for the presence of human CD45+ hematopoietic cells, myeloid cells (hCD45+CD33+), and B lymphoid cells (hCD45+CD19+) cells. The right plots are gated on human CD45+ cells.
B. Summary of long-term (> 12 weeks) bone marrow engraftment of CD90/CD45RA subpopulations
C. Bone marrow engraftment per 100 transplanted cells
The percent human chimerism (left) and percent human myeloid cells (right) per 100 transplanted cells is indicated for each engrafted mouse. Each circle or triangle represents an individual mouse and the bar indicates the average. On average, CD90+CD45RA- mice developed 9 fold more human chimerism than CD90-CD45RA- mice, and this difference was statistically significant (p=0.001). The 9 fold difference in percent human myeloid cells approached, but did not achieve statistical significance (p=0.07).
D. Summary of long-term (>11 weeks) bone marrow engraftment of limiting numbers (<100 cells) of CD90/CD45RA subpopulations
50 or 70 double FACS-purified CD90+CD45RA- or CD90-CD45RA- cells were transplanted into NOG newborn mice as described. At least 11 weeks later bone marrow was analyzed by flow cytometry for the presence of human CD45+ hematopoietic cells, myeloid cells (hCD45+CD33+), B cells (hCD45+CD19+) cells, and T cells (hCD45+CD3+). In 1 out of 5 mice transplanted with CD90-CD45RA- cells, a small population of T cells (0.2%) was detected in the bone marrow, in the absence of myeloid and B cells. The difference in successful engraftment was statistically significant (p=0.008).
Analysis of the spleens of CD90+CD45RA- and CD90-CD45RA- transplanted mice identified human CD45+ cells consisting of rare myeloid cells, numerous B cells, and occasional T cells. No human cells were detected in the spleens of mice transplanted with CD90-CD45RA+ cells. Significant numbers of T cells were detected in only a subset of mice transplanted with either engrafting population (Supplementary Figure 2). Determining splenic engraftment per 100 transplanted cells revealed that CD90+CD45RA- engrafted mice averaged 7 fold greater human chimerism than the CD90-CD45RA- engrafted mice, and this difference was statistically significant; however, no statistically significant difference was detected in the T cell percentage (Supplementary Figure 2B). Finally, bone marrow from mice engrafted with CD90-CD45RA- cells was found to contain GPA-positive human erythroid cells and CD61/CD41-positive human platelets (data not shown), indicating that these cells are multipotent.
To investigate the minimum number of cells required for successful engraftment, 50 or 70 CD90-CD45RA- or CD90+CD45RA- cells were double FACS-purified from 2 independent cord blood samples and transplanted into NOG mice. At least 11 weeks later, bone marrow was analyzed for human engraftment. All mice (n=4) transplanted with CD90+CD45RA- cells contained human myeloid and B cells, while no mice (n=5) transplanted with CD90-CD45RA- cells did (Figure 5D). This difference was statistically significant with p=0.008. One of the CD90-CD45RA- transplanted mice did contain a small population (0.2%) of human T cells, indicating that it must have engrafted at an early time point. To directly investigate early engraftment, 50 double FACS-purified cells were transplanted into NOG newborn mice. 4 weeks later, bone marrow of all transplanted mice, both CD90+CD45RA- (n=2) and CD90-CD45RA- (n=2), contained human myeloid and B cells.
In summary, both the CD90+CD45RA- and CD90-CD45RA- subpopulations are capable of establishing long-term in vivo multipotent human hematopoiesis, with similar percentages of myeloid and lymphoid cell production. However, the CD90-CD45RA-cells have a lower engraftment capacity as they require transplantation of more cells for long-term engraftment and generate fewer human cells per cell-equivalent transplant.
In Vivo Analysis of Human CD34+ Cells Identifies a Hierarchy Among the CD90/CD45RA Subpopulations
In order to assess the hierarchical relationships among the CD90/CD45RA subpopulations in vivo, human CD34+ progenitor cells from the bone marrows of engrafted mice were analyzed 12 weeks after transplantation. In mice transplanted with CD90+CD45RA- cells the bone marrow contained, on average, 6.4% human CD34+ cells compared to 1.3% human CD34+ cells in mice transplanted with CD90-CD45RA-cells (Figure 6A). CD34+ engraftment per 100 transplanted cells averaged 8 fold greater in CD90+CD45RA- engrafted mice than CD90-CD45RA- engrafted mice, and this difference was statistically significant (Figure 6B). This 8 fold statistically significant difference was also present when comparing the percentage of Lin-CD34+ cells in total bone marrow (data not shown). Within the engrafted Lin-CD34+ fraction, there were no differences between CD90+CD45RA- and CD90-CD45RA- engrafted mice with respect to the percentages of CD34+CD38+ or CD34+CD38- cells (Figure 6A). Thus, CD90-CD45RA- cells appear to be able to generate progenitor cells in similar proportions to CD90+CD45RA- cells, but are less efficient in doing so in vivo.
Figure 6. In Vivo Analysis of Human CD34+ Cells Identifies a Hierarchy Among the CD90/CD45RA Subpopulations.
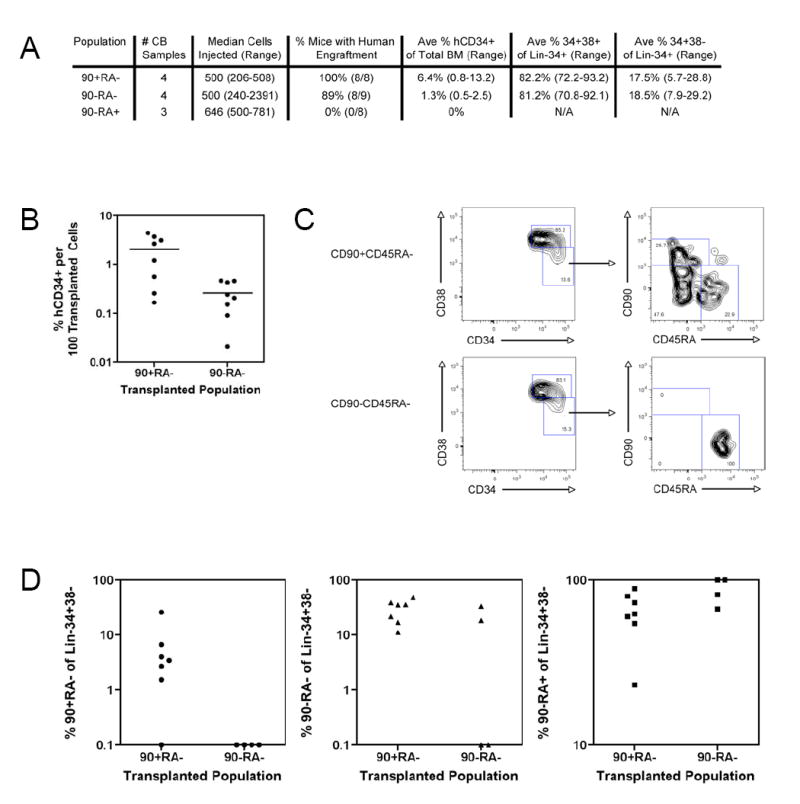
A. Summary of long-term (>12 weeks) human CD34+ bone marrow engraftment of CD90/CD45RA subpopulations
B. Bone marrow human CD34+ engraftment per 100 transplanted cells
The percentage of human CD34+ cells in total bone marrow per 100 transplanted cells is indicated for each engrafted mouse. Each circle represents an individual mouse and the bar indicates the average. On average, CD90+CD45RA- mice contained 8 fold more human CD34+ cells than CD90-CD45RA- mice, and this difference was statistically significant (p=0.01).
C. Analysis of CD90/CD45RA expression on Lin-CD34+CD38- bone marrow cells in CD90/CD45RA transplanted mice
500 FACS-purified CD90+CD45RA- cells (top panels) and CD90-CD45RA- cells (bottom panels) were transplanted into NOG newborn mice as described. 12 weeks later bone marrow was analyzed by flow cytometry for the expression of lineage markers, CD34, CD38, CD90, and CD45RA. The left plots are gated on Lin-CD34+ cells, and the right plots are gated on Lin-CD34+CD38- cells.
D. CD90/CD45RA subpopulations within engrafted bone marrow
The percentage of CD90+CD45RA- (left), CD90-CD45RA- (middle), and CD90-CD45RA+ (right) cells out of Lin-CD34+CD38- bone marrow cells from mice engrafted with CD90+CD45RA- and CD90-CD45RA- cells is indicated. Each circle, triangle, or square represents an individual mouse. Only mice with greater than 10 Lin-CD34+CD38- cells were included (n=7 transplanted with CD90+CD45RA- cells and n=4 transplanted with CD90-CD45RA- cells).
Human Lin-CD34+CD38- cells in the bone marrow of engrafted mice were also analyzed for the expression of CD90 and CD45RA. All three CD90/CD45RA subpopulations were detected in the bone marrow of mice transplanted with CD90+CD45RA- cells; however, only the two CD90- subpopulations were detected in mice transplanted with CD90-CD45RA- cells (Figure 6 C, D). In some mice transplanted with CD90-CD45RA- cells, only CD90-CD45RA+ cells were detected. Together, these data establish an in vivo differentiation hierarchy among Lin-CD34+CD38- cord blood cells proceeding from CD90+CD45RA- to CD90-CD45RA- to CD90-CD45RA+ cells.
Enrichment of Secondary Transplant Ability in the CD90+CD45RA- Subpopulation
Self-renewal is the key property distinguishing HSC from MPP, and is defined by the ability to sustain long-term hematopoiesis and generate successful secondary transplants. Since both the CD90+CD45RA- and CD90-CD45RA- subpopulations demonstrated the ability to establish long-term in vivo multipotent hematopoiesis, we next assessed their ability to generate successful secondary transplants. Human CD34+ cells were purified from whole bone marrow of primary engrafted mice and equal numbers of CD34+ cells were transplanted into NOG newborn mice. Bone marrows from these secondary recipients were analyzed at least 10 weeks after transplantation. In one experiment, 130,000 human CD34+ cells were transplanted into secondary recipients. Human CD45+ cells and human myeloid cells were detected in 8 of 8 mice (100%) from CD90+ primary transplants, but only 2 of 5 mice (40%) from CD90- primary transplants (Figure 7 A,B). In a second experiment, 70,000 human CD34+ cells were transplanted into secondary recipients. Human CD45+ cells and human myeloid cells were detected in 4 of 4 mice (100%) from CD90+ primary transplants, but only 1 of 3 mice (40%) from CD90- primary transplants (Figure 7 A,C). This difference, 12 of 12 (100%) for CD90+ versus 3 of 8 (37.5%) for CD90-, was statistically significant with p=0.004. These data indicate that the CD90+CD45RA- subpopulation is enriched for the ability to generate successful secondary transplants compared to the CD90-CD45RA- subpopulation.
Figure 7. Enrichment of Secondary Transplant Ability in the CD90+CD45RA Subpopulation.
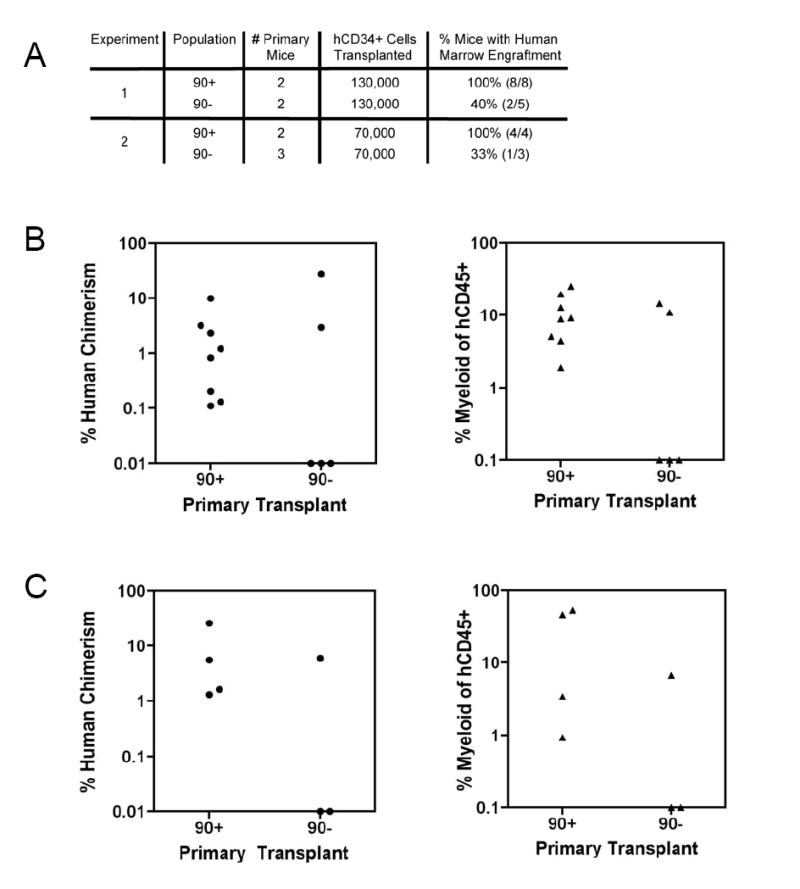
A. Summary of long-term (> 10 weeks) bone marrow engraftment in secondary transplants from mice engrafted with either CD90+ or CD90- subpopulations
The difference in successful secondary engraftment, 12 of 12 (100%) for CD90+ versus 3 of 8 (37.5%) for CD90-, was statistically significant with p=0.004.
B. Bone marrow engraftment in secondary recipients from experiment 1
Primary mice were transplanted with 2000 cells of the indicated population. The percent human chimerism (left) and percent myeloid cells of total human CD45+ cells (right) is indicated for each secondary mouse. Each circle or triangle represents an individual mouse.
C. Bone marrow engraftment in secondary recipients from experiment 2
Primary mice were transplanted with 500 cells of the indicated population. The percent human chimerism (left) and percent myeloid cells of total human CD45+ cells (right) is indicated for each secondary mouse. Each circle or triangle represents an individual mouse.
DISCUSSION
HSC are defined by two key functional properties: (1) multipotency, defined as the ability to form all differentiated blood cells, and (2) long-term self-renewal, defined as the ability to give rise to progeny identical to the parent through cell division. It is this property of self-renewal that distinguishes HSC from multipotent progenitors, and is experimentally demonstrated through the ability to generate successful secondary transplants. We show here that both CD90+CD45RA- and CD90-CD45RA- cord blood cells are able to establish long-term multipotent hematopoiesis in vivo, and that the CD90+CD45RA- subpopulation is enriched for the ability to generate successful secondary transplants. We conclude that the CD90+CD45RA- subpopulation contains HSC, while the CD90-CD45RA- subpopulation contains candidate multipotent progenitors. This represents the first identification and prospective isolation of a population of candidate human multipotent progenitors.
Both the CD90+CD45RA- and CD90-CD45RA- cells are able to establish multipotent long-term human hematopoiesis in vivo; however, the CD90-CD45RA-subpopulation requires more cells to accomplish this as indicated by the failure of 50 and 70 cell transplants to long-term engraft, unlike the CD90+CD45RA- subpopulation which engrafts long-term with as few as 10 cells. There are three possibilities to explain these results. First, it is possible that the CD90-CD45RA- cells are contaminated with infrequent CD90+CD45RA- cells, as CD90 expression is present on a continuum and not separable into discrete subpopulations. The second alternative is that the CD90-CD45RA- cells do contain HSC, but at a lower frequency than the CD90+CD45RA-cells. Third, the CD90-CD45RA- cells may have reduced capacity to home to the bone marrow, which could account for the in vivo functional differences observed, although this would not account for the differences in methylcellulose colony replating. Ultimately, our data do not discriminate among these three possibilities. Nevertheless, the fact that 50 CD90-CD45RA- cells show myeloid and B lymphoid engraftment at 4 weeks, but not 12 weeks, indicates that this fraction contains non-HSC multipotent cells. Thus, we have demonstrated that CD90-CD45RA- cells are multipotent, exhibit a reduced and incomplete capacity for self-renewal, and lie downstream of CD90+CD45RA- cells in the hematopoietic hierarchy. We conclude that CD90-CD45RA- cells are a candidate multipotent progenitor.
Hematopoiesis proceeds through an organized hierarchy in which lineage potential becomes increasingly restricted and a given population can only give rise to downstream populations. We investigated the hierarchical relationships between the CD90/CD45RA subpopulations of Lin-CD34+CD38- cord blood and found that both in vitro and in vivo, the CD90+CD45RA- population gives rise to itself and both the CD90-CD45RA- and CD90-CD45RA+ subpopulations. CD90-CD45RA- cells do not give rise to CD90+ cells, but can form both CD90- subpopulations. In vitro the CD90-CD45RA+ cells give rise principally to itself only. These results establish a hierarchy among Lin-CD34+CD38- cord blood cells in which CD90+CD45RA- cells are upstream of CD90-CD45RA- cells, which are upstream of CD90-CD45RA+ cells (Supplementary Figure 4). Several investigators have reported long-term engraftment with Lin-CD34+CD38-/lo cord blood cells. As CD38 exhibits a continuum of expression on Lin-CD34+ cells, it is possible that some CD38lo cells were included in our purified subpopulations. Additional xenotransplantation experiments will be necessary to determine the function and hierarchical relationship of Lin-CD34+CD38lo cord blood cells to our defined subpopulations.
CD90 and CD45RA identify a third population within the Lin-CD34+CD38-fraction of cord blood and bone marrow, the CD90-CD45RA+ subpopulation. The experiments outlined here to identify the differentiation and self-renewal potential of this population were unsuccessful. Transplantation with up to 900 of these cells does not result in any circulating human hematopoietic cells at 4 weeks after transplantation, and there are no detectable human cells in the bone marrow at 12 weeks. It is possible that in vivo functional engraftment of this population will require transplantation of many more cells than performed here, which was limited by the lower frequency of this population in cord blood. Furthermore, these cells have extremely poor methylcellulose colony forming ability, yielding only 6 colonies from 180 plated cells, suggesting that they possess limited myeloid differentiation potential. These cells also did not proliferate in liquid culture under conditions able to promote the growth of the other two subpopulations. It is interesting to note, that cells with this immunophenotype can be found within the bone marrow of mice engrafted with either the CD90+CD45RA- or CD90-CD45RA- subpopulation. Thus, it is likely that they contribute to ongoing human hematopoiesis, but at this time cannot be placed within the hematopoietic hierarchy (Supplementary Figure 4).
Numerous xenotransplantation experiments have reported the successful enrichment of human HSC activity in Lin-CD34+CD38-/lo fractions of human hematopoietic progenitors through the demonstration of long-term multipotent engraftment and successful secondary transplantation (Dick et al., 2001; McKenzie et al., 2006). In published reports, successful secondary engraftment has required primary transplantation of large numbers of cells, minimally thousands and usually many more. We report here long-term in vivo human engraftment and successful secondary engraftment with transplantation of 500 purified Lin-CD34+CD38-CD90+CD45RA-cord blood cells. The major differences between our results and previous reports are: (1) the use of the NOG newborn mice, which appear to be well-suited for human hematopoietic engraftment, and (2) the combination of Lin-CD34+CD38-CD90+CD45RA- markers for HSC purification. With this immunophenotype and xenotransplantation assay, we have directly isolated cord blood HSC activity to fewer cells than in previous reports.
Implications for Human Acute Myeloid Leukemia
Analogous to normal hematopoiesis, human acute myeloid leukemia (AML) is organized as a hierarchy initiated by leukemia stem cells (LSC) that are able to self-renew and give rise to all the cells within the leukemia (Tan et al., 2006; Wang and Dick, 2005). In a series of xenotransplantation experiments, Dick and colleagues first demonstrated the existence of human AML LSC and localized them to the Lin-CD34+CD38- fraction of AML (Bonnet and Dick, 1997; Lapidot et al., 1994). Based on these observations, they have proposed a model in which HSC are the cell of origin for AML LSC (Wang and Dick, 2005). However, subsequent experiments indicated that AML LSC, unlike HSC, do not express CD90 (Blair et al., 1997; Miyamoto et al., 2000). There are two hypotheses to account for this difference: (1) AML LSC are indeed derived from HSC, but have aberrantly lost expression of CD90, or (2) AML LSC do not derive from HSC but instead come from a downstream progenitor that lacks expression of CD90.
Evidence supporting the second hypothesis comes from our previous studies of AML1-ETO translocation-associated AML in atom bomb survivors from Hiroshima (Miyamoto et al., 2000). Similar to other investigators, we found that the AML LSC were contained in the Lin-CD34+CD38-CD90- fraction (Blair et al., 1997; Miyamoto et al., 2000). However, when the bone marrow of long-term disease-free survivors was examined, the AML1-ETO translocation was detected in Lin-CD34+CD38-CD90+ non-leukemic HSC. This demonstrates that pre-leukemic genetic changes can take place within HSC, but ultimate transformation to AML LSC requires additional mutations that do not occur in HSC, but instead take place in a CD90- downstream population. We now present evidence identifying this CD90- downstream population as MPP.
Why does this matter? If the normal counterpart to long-term self-renewing AML LSC is not capable of long-term self-renewal itself, then AML LSC must have undergone mutational or epigenetic activation of a self-renewal pathway. These changes, when identified, become potential targets for therapeutic intervention to eradicate the LSC. Evidence for aberrant activation of self-renewal in LSC comes from our previous studies of human blast crisis CML, where normally non-self-renewing cells transform into LSC in part through activation of the Wnt/beta-catenin pathway (Jamieson et al., 2004). Through comparisons between AML LSC and the newly identified MPP, we hope to identify genetic and/or epigenetic events than lead to the transformation of MPP into AML LSC.
EXPERIMENTAL PROCEDURES
Human Samples
Normal human bone marrow mononuclear cells were purchased from AllCells Inc. (Emeryville, CA). Human cord blood was collected from placentas and/or umbilical cords obtained from the Stanford Medical Center, according to an IRB-approved protocol (Stanford IRB# 4637). Mononuclear cells were prepared using Ficoll-Paque Plus (GE Healthcare, Fairfield, CT), and cryopreserved in 90% FBS/10% DMSO. All experiments were conducted with cryopreserved cord blood cells that were thawed and washed with IMDM containing 10% FBS. In some cases, CD34+ cells were enriched using MACS (Miltenyi Biotec, Germany) or Robosep (Stem Cell Technologies, Canada) immunomagnetic beads.
Flow Cytometry Analysis and Cell Sorting
A panel of antibodies was used for analysis and sorting of progenitor subpopulations, as well as lineage analysis of human chimerism/engraftment, and used to stain cell suspensions (Supplementary Methods). Cells were either analyzed or sorted using a FACSAria cytometer (BD Biosciences). Analysis of flow cytometry data was performed using FlowJo Software (Treestar, Ashland, OR). Raw engraftment data is provided in Supplementary Figure 3. Statistical analysis using Student’s t-test or Fisher’s exact test was performed with Microsoft Excel and/or GraphPad Prism (San Diego, CA) software.
In Vitro Assays: Cytology, Methylcellulose, and Liquid Culture
For cytologic analysis, sorted cells were centrifuged onto slides using a Shandon Cytocentrifuge 4 (Thermo Scientific, Waltham, MA), and stained with Wright-Giemsa. Photomicrographs were taken using a 100x objective under oil.
Methylcellulose colony formation was assayed by clone-sorting single cells into individual wells of a 96-well plate, each containing 100 ul of complete methylcellulose (Methocult GF+ H4435, Stem Cell Technologies). Plates were incubated for 12-14 days at 37°C, then scored based on morphology. All colonies were harvested, dissociated by resuspending in sterile PBS, and replated into individual wells of a 24-well plate, each containing 500 ul of complete methylcellulose. Plates were incubated for 12-14 days at 37°C, after which replating was determined by assessing colony formation. Statistical analysis using Student’s t-test was performed with Microsoft Excel and/or GraphPad Prism (San Diego, CA) software.
In vitro proliferation was assayed by clone-sorting single cells into individual wells of a 96-well plate, each containing 100 ul of StemSpan media (Stem Cell Technologies), supplemented with 40 ug/ml human LDL (Sigma-Aldrich) and cytokines (R&D Systems, Minneapolis, MN): 100 ng/ml Flt-3 ligand, 100 ng/ml SCF, 50 ng/ml TPO, 20 ng/ml IL-3, and 20 ng/ml IL-6. Plates were incubated for 14 days at 37°C, after which live cells were counted by trypan blue exclusion. For in vitro differentiation assays, cells were sorted in bulk into this same culture media and incubated for 3-4 days at 37°C, after which cells were harvested and analyzed by flow cytometry. Statistical analysis using Student’s t-test was performed with Microsoft Excel and/or GraphPad Prism (San Diego, CA) software.
Mouse Transplantation
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (NOG) were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in a Specific Pathogen-Free environment per Stanford Administrative Panel on Laboratory Animal Care guidelines (Protocol 10725). P0-P2 newborn pups were conditioned with 100 rads of gamma irradiation up to 24 hours prior to transplantation (Ishikawa et al., 2005). Desired cells were resuspended in 20-40 ul of PBS containing 2% FBS and transplanted intravenously via the anterior facial vein using a 30 or 31 gauge needle. For secondary transplants, human CD34+ bone marrow cells from primary engrafted mice were enriched using MACS immunomagnetic beads (Miltenyi Biotec), and transplanted into newborn NOG recipients.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Libuse Jerebek for excellent lab management, Adriane Mosely for animal husbandry, and Chrissy Muscat for antibody preparation. We acknowledge Laurie Ailles and the members of the Weissman lab for excellent discussion. R.M. is supported by a grant from the Walter and Idun Y. Berry Foundation. R.M. and C.Y.P have no conflicts of interest to disclose. I.L.W. was a member of the scientific advisory board of Amgen and owns significant Amgen stock. I.L.W. co-founded and consulted for Systemix, is a co-founder and director of Stem Cells Inc., and co-founded Cellerant, Inc. This research is supported by National Institutes of Health grant R01CA86017 to I.L.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci U S A. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–3112. [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman JD, Lapidot T, Wang JC, Doedens M, Shultz LD, Lansdorp P, Dick JE, Eaves CJ. Kinetic evidence of the regeneration of multilineage hematopoiesis from primitive cells in normal human bone marrow transplanted into immunodeficient mice. Blood. 1997;89:4307–4316. [PubMed] [Google Scholar]
- Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177:1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JE, Guenechea G, Gan OI, Dorrell C. In vivo dynamics of human stem cell repopulation in NOD/SCID mice. Ann N Y Acad Sci. 2001;938:184–190. doi: 10.1111/j.1749-6632.2001.tb03588.x. [DOI] [PubMed] [Google Scholar]
- Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- Hogan CJ, Shpall EJ, Keller G. Differential long-term and multilineage engraftment potential from subfractions of human CD34+ cord blood cells transplanted into NOD/SCID mice. Proc Natl Acad Sci U S A. 2002;99:413–418. doi: 10.1073/pnas.012336799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Mazurier F, Gan OI, McKenzie JL, Doedens M, Dick JE. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood. 2004;103:545–552. doi: 10.1182/blood-2003-05-1558. [DOI] [PubMed] [Google Scholar]
- McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- McKenzie JL, Gan OI, Doedens M, Wang JC, Dick JE. Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat Immunol. 2006;7:1225–1233. doi: 10.1038/ni1393. [DOI] [PubMed] [Google Scholar]
- Michallet M, Philip T, Philip I, Godinot H, Sebban C, Salles G, Thiebaut A, Biron P, Lopez F, Mazars P, et al. Transplantation with selected autologous peripheral blood CD34+Thy1+ hematopoietic stem cells (HSCs) in multiple myeloma: impact of HSC dose on engraftment, safety, and immune reconstitution. Exp Hematol. 2000;28:858–870. doi: 10.1016/s0301-472x(00)00169-7. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci U S A. 2000;97:7521–7526. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Murray L, Chen B, Galy A, Chen S, Tushinski R, Uchida N, Negrin R, Tricot G, Jagannath S, Vesole D, et al. Enrichment of human hematopoietic stem cell activity in the CD34+Thy-1+Lin- subpopulation from mobilized peripheral blood. Blood. 1995;85:368–378. [PubMed] [Google Scholar]
- Negrin RS, Atkinson K, Leemhuis T, Hanania E, Juttner C, Tierney K, Hu WW, Johnston LJ, Shizurn JA, Stockerl-Goldstein KE, et al. Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant. 2000;6:262–271. doi: 10.1016/s1083-8791(00)70008-5. [DOI] [PubMed] [Google Scholar]
- Peault B, Weissman I, Baum C. Analysis of candidate human blood stem cells in “humanized” immune-deficiency SCID mice. Leukemia. 1993;7(Suppl 2):S98–101. [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Sutherland HJ, Eaves CJ, Eaves AC, Dragowska W, Lansdorp PM. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989;74:1563–1570. [PubMed] [Google Scholar]
- Tan BT, Park CY, Ailles LE, Weissman IL. The cancer stem cell hypothesis: a work in progress. Lab Invest. 2006;86:1203–1207. doi: 10.1038/labinvest.3700488. [DOI] [PubMed] [Google Scholar]
- Vose JM, Bierman PJ, Lynch JC, Atkinson K, Juttner C, Hanania CE, Bociek G, Armitage JO. Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with recurrent indolent non-Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2001;7:680–687. doi: 10.1053/bbmt.2001.v7.pm11787531. [DOI] [PubMed] [Google Scholar]
- Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


