Abstract
Caspofungin acetate is an antifungal antibiotic that inhibits synthesis of 1,3-β-d-glucan, an essential component of the fungal cell wall. While caspofungin causes cell death in yeasts and dimorphic fungi such as Candida albicans, its effect on Aspergillus fumigatus is less well understood. We used the fluorescent dyes 5,(6)-carboxyfluorescein diacetate (CFDA) and bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC), which stain live and dead cells, respectively, to further characterize the antifungal activity of caspofungin. For comparison, compounds whose mode of action was either fungistatic (fluconazole, itraconazole) or fungicidal (amphotericin B) were also evaluated. A correlation between caspofungin-induced loss of viability, decreased CFDA staining, and increased DiBAC staining was established first with C. albicans. For A. fumigatus, caspofungin caused similar dye-staining changes, which were quantified by fluorimetric analysis of stained hyphae grown in a medium that promoted dispersed growth. The minimum concentration of caspofungin required to produce these changes also decreased the level of growth-dependent reduction of the indicator dye Alamar Blue. We observed a differential effect of caspofungin as a function of cell position: 88% of apical cells and 61% of subapical branching cells failed to stain with the viable dye CFDA, but only 24% of subapical cells were unstained. Complementary results were seen with germlings from DiBAC-stained, caspofungin-treated cultures. Extended incubation of A. fumigatus with a single dose of caspofungin affected the same proportion of apical and subapical branching cells for up to 72 h. The dye-staining patterns illustrate that the cells at the active centers for new cell wall synthesis within A. fumigatus hyphae are killed when they are exposed to caspofungin.
Caspofungin, the first clinically used echinocandin, is a member of a new class of antifungal antibiotics that inhibit the synthesis of 1,3-β-d-glucan. The echinocandins are potent against many pathogenic fungi while maintaining an excellent safety profile. Inhibition of cell wall synthesis represents a novel mechanism of action compared to those of existing antifungal agents, which target fungal cell membranes. Amphotericin B (AMB), a polyene, is fungicidal in vitro and in vivo against Candida albicans and Aspergillus fumigatus, while the azoles fluconazole (FLC) and itraconazole (ITC) demonstrate fungistatic activities against these organisms.
The consequences of inhibiting yeast cell wall formation, specifically glucan synthesis, are cell morphology changes and loss of viability. Treatment of C. albicans with echinocandin caused growing cells to lyse (13), and caspofungin has been shown to be fungicidal against numerous C. albicans isolates (6). Ernst et al. (19) reported that C. albicans cells treated with caspofungin at a level that inhibits growth by 80% (MIC80) were highly enlarged compared to control cells; when treated with caspofungin at the 100% inhibitory level (MIC100), all cells lysed. For filamentous fungi, inhibition with caspofungin results in profound changes in the growth, morphology, and cell wall structure of hyphae (26). Caspofungin and other echinocandins have been shown to inhibit 1,3-β-d-glucan synthesis activity in the filamentous ascomycetes A. fumigatus (9, 26) and Aspergillus nidulans (25). The FKS gene encodes a subunit of the 1,3-β-d-glucan synthase complex in several fungal species, including Saccharomyces cerevisiae (18), A. nidulans (25), C. albicans (17), and Cryptococcus neoformans (38). Beauvais et al. (9) have shown that the A. fumigatus Fks protein localizes to the hyphal apex. Likewise, fluorescence is most intense at the tips of hyphae stained with a β-glucan-specific fluorochrome (aniline blue). In A. nidulans, new cell wall formation occurs exclusively at the hyphal apices (31), and mutations affecting this process cause aberrant growth (31, 40).
Caspofungin acetate has been approved for use in the treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies. Caspofungin has been shown to have efficacy in animal models of disseminated (1, 2, 11) and pulmonary (33; E. M. Bernard, T. Ishimaur, and D. Armstrong, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F39, 1996) aspergillosis, and liquid broth microdilution assays demonstrate growth reduction resulting from caspofungin treatment (20, 35). However, it has not been clear that caspofungin can kill A. fumigatus cells. In both animal models and in vitro cultures, echinocandins often produce little to no reduction in the numbers of A. fumigatus CFU (20, 33, 34). Characterization of the nature of the activity of echinocandins against A. fumigatus is further confounded by the inability to quantify changes in cell mass or observe individual germ tubes in liquid culture due to the intertwined, filamentous growth of the organism.
A recent report described the use of fluorescent probes to detect the fungicidal activity of AMB against C. albicans (28). At concentrations of AMB that significantly reduce the number of CFU, the level of fluorescent staining with the dye 5,(6)-carboxyfluorescein diacetate (CFDA), which stains live cells, decreased, while the level of staining with the dye bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC), which stains dead cells, increased. The esterase activity of viable cells cleaves the acetate moieties from CFDA to produce free carboxyfluorescein, which is retained in cells whose membranes are intact (36). DiBAC is a member of the oxonol class of dyes that exhibit intense fluorescence upon binding to phospholipids (8). Normal membrane potential, which collapses with mortal injury or cell death, prevents access of DiBAC to the inside of the cell. We sought to further understand the activity of caspofungin against A. fumigatus using these dyes. Our initial experiments performed with C. albicans established a strong correlation between caspofungin-induced changes in dye staining and reductions in the number of viable CFU. With minor modifications, including addition of the polyacrylate Junlon PW110 (Junlon) to the medium to promote dispersed growth, we were able to stain germlings of A. fumigatus strain MF5668 with CFDA and DiBAC and detect fluorescence qualitatively by microscopy and quantitatively by liquid broth microtitrations. Our results demonstrate that caspofungin can cause lysis of A. fumigatus cells. Direct counting of stained caspofungin-treated germlings demonstrated a preferential effect of the drug on cells at the tips and branch points of growing A. fumigatus hyphae.
(This work was presented in part at the 40th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Ontario, Canada, 19 September 2000.)
MATERIALS AND METHODS
Drugs.
Caspofungin was dissolved in sterile distilled water. AMB (Sigma Chemical Co., St. Louis, Mo.), FLC (Pfizer, Groton, Conn.), and ITC (Janssen Pharmaceutica, Titusville, N.J.) were dissolved in 100% dimethyl sulfoxide.
Media.
C. albicans cultures were grown in RPMI 1640 medium with l-glutamine buffered to pH 7.0 with 0.165 M MOPS [3-(N-morpholino)propanesulfonic acid; BioWhittaker, Boston, Mass.]. A. fumigatus cultures were grown in RPMI 1640 medium containing MOPS (pH 7.0) and 0.15% (wt/vol) Junlon (Nihon Junyaku Co., Ltd., Tokyo, Japan). Briefly, powdered RPMI 1640 with l-glutamine (Gibco BRL, Bethesda, Md.) was resuspended in distilled H2O, MOPS (Fisher Scientific) was added to a final concentration of 0.165 M, the pH was adjusted to 7.0, the volume was brought to 800 ml, and the medium was sterile filtered. To prepare Junlon, 1.5 g was added to 200 ml of distilled H2O and heated in a microwave until no discernible clumps were seen. The Junlon was autoclaved and cooled to room temperature before the RPMI 1640 medium was added.
Organisms and preparation of inocula.
C. albicans strain MY1055 from the culture collection of Merck (Rahway, N.J.) was spread onto YPAD (1% yeast extract, 2% peptone, 2% dextrose, 4 mg of adenine per ml) plates (Difco, Detroit, Mich.), and the plates were incubated for 2 to 4 days at 35°C. A. fumigatus strain MF5668 (ATCC 13073) is a clinical isolate from a human pulmonary lesion (1). Conidia were inoculated on Sabouraud dextrose agar (SDA) slants (BBL, Cockeysville, Md.) and grown at 37°C, with the caps ajar, for 5 to 7 days to allow sporulation. Five milliliters of phosphate-buffered saline (Gibco BRL) containing 0.05% (vol/vol) Tween 20 was added, and the conidia were harvested by gently rubbing the surface with a sterile cotton swab and decanting the liquid. A stock of 1 × 106 to 5 × 106 conidia/ml was prepared by adding drops of the concentrated spore suspension to 5 ml of sterile saline (Becton Dickinson) until the optical density (OD), read in a MicroScan turbidity meter (Dade Behring, West Sacramento, Calif.), equaled that of a 0.5 McFarland standard (Remel, Lenexa, Kans.).
Cell growth and drug treatment.
Several colonies of C. albicans strain MY1055 were inoculated from YPAD plates into 20 ml of buffered RPMI 1640 medium and grown to saturation (ca. 40 h) at 37°C with shaking (220 rpm). The cells were subcultured to an initial A600 of 0.002 in 65 ml of fresh medium, and incubation was continued until the cultures reached a density of 1× 106 to 2 × 106 cells/ml (determined by counting with a hemocytometer; ca. 9 h). Aliquots were removed, serially diluted in buffered RPMI 1640 medium, and spread onto SDA plates (BBL) to determine the number of CFU per milliliter. Compound (caspofungin, AMB, or FLC) or vehicle was added, and incubation was continued for 15 h. Following treatment, samples from each culture were evaluated for the number of CFU per milliliter or the total cell count. Cells from each 65-ml culture were collected by centrifugation (10,000 × g, 10 min, 4°C) and brought to 6.5 ml with buffered RPMI 1640 medium; 1.5-ml aliquots were transferred to microcentrifuge tubes, and cells were collected by centrifugation (9,500 × g, 5 min, room temperature). For DiBAC staining, cells were resuspended in 1.5 ml of 0.1 M MOPS at pH 7.0 (MOPS 7). For CFDA staining, cells were washed two times with MOPS 7 and resuspended in 1.5 ml of 0.1 M MOPS-50 mM citric acid at pH 3.0 (MOPS 3).
A. fumigatus MF5668 conidia in sterile saline suspensions were counted with a hemocytometer and inoculated into 80 ml of buffered RPMI 1640 medium containing 0.15% (wt/vol) Junlon (RPMI-Junlon) to an initial density of 105 conidia/ml. The culture was incubated for 14 h at 37°C with shaking (220 rpm). Caspofungin, AMB, ITC, or vehicle was then added, and incubation was continued. To estimate growth inhibition, RPMI-Junlon cultures (20 ml) containing 10% (vol/vol) Alamar Blue (Trek Diagnostics, Westlake, Ohio) were evaluated in parallel. Cell-free supernatants were prepared from 1-ml aliquots by centrifugation (3,200 × g, 5 min, 22°C), the absorbance at 603 nm was measured, and the Alamar Blue reduction index was calculated from the formula OD603 untreated/OD603 treated. For staining with fluorescent probes, duplicate 15-ml aliquots from each RPMI-Junlon culture were collected by centrifugation (3,200 × g, 5 min, 4°C), which produced three phases: a supernatant containing no Junlon and no detectable hyphae, a Junlon layer containing a high density of trapped germlings, and a pellet containing larger clumps of hyphae. Analysis of total protein (see below) in the three layers illustrated that 60% of the total protein was in the pellet, with the remaining 40% in the Junlon layer (data not shown). The Junlon layer was transferred to a new tube, and buffer (MOPS 3 for CFDA staining, MOPS 7 for DiBAC staining) was added to a final volume of 5 ml.
Staining with fluorescent probes.
CFDA staining was performed with cell suspensions in MOPS 3 by adding CFDA (Molecular Probes, Eugene, Oreg.) from a 5-mg/ml stock in 100% dimethyl sulfoxide to a final concentration of 50 μg/ml. Samples were incubated in the dark at 37°C for 45 min with gentle agitation and then stored on ice until analysis. For DiBAC staining, cell suspensions in MOPS 7 were used. DiBAC (Molecular Probes) from a 1-mg/ml stock in 100% ethanol was added to a final concentration of 2 μg/ml; samples were incubated in the dark with shaking at room temperature (ca. 22°C) for 1 h, washed twice with MOPS 7, and stored on ice. Washing of C. albicans samples (1.5-ml aliquots) was performed by centrifugation at 9,500 × g for 5 min; A. fumigatus germlings in the Junlon layer were collected and washed by centrifugation at 3,200 × g for 10 min. To account for background fluorescence in DiBAC-stained A. fumigatus samples, an aliquot of uninoculated RPMI-Junlon medium was processed in parallel and the fluorescence value was subtracted.
Fluorometry.
Fluorescence was measured with a Fluoroskan II spectrofluorometer (Labsystems, Helsinki, Finland) by using a fluorescein isothiocyanate (FITC) filter pair (excitation wavelength = 485 nm; emission wavelength = 538 nm) and 96-well Optiplates (Packard, Meriden, Conn.). Samples of each stained cell suspension (400 μl) were added in triplicate to wells of the first Optiplate row and were serially diluted twofold to the last row of the plate in 200 μl of either MOPS 7 (DiBAC-stained samples) or MOPS 3 (CFDA-stained samples).
Normalization.
C. albicans fluorescence values were normalized to cell density. Plots of fluorescence versus cell density yielded curves whose values for CFDA fluorescence per 106 cells or for DiBAC fluorescence per 107 cells were near the middle of the linear portion of the curve. The cells in an aliquot of each CFDA- or DiBAC-stained C. albicans culture were counted in a hemocytometer, and the relative fluorescence per 106 cells (CFDA) or 107 cells (DiBAC) were recorded. For A. fumigatus cultures, fluorescence values were normalized to the amount of cellular protein. To determine protein concentration, stained germlings were disrupted with 0.5-mm-diameter glass beads (BioSpec Products, Bartlesville, Okla.) with a Bead-Beater homogenizer (BioSpec Products). Samples (1 ml) from each culture were dispensed into 2-ml screw-cap tubes (Sarstedt, Numbrecht, Germany), glass beads were added to fill the tube, and germlings were broken with three 30-s bursts at 5,000 rpm. The homogenates were collected by centrifugation at 800 × g for 5 min at 4°C, and the protein concentration was determined with MicroBCA reagents (Pierce, Rockford, Ill.), with a standard of bovine serum albumin, according to the recommendation of the manufacturer. Fluorescence values were normalized per 1 mg of protein per ml for both dyes.
A cell staining index was used to measure the fold change in fluorescence due to drug treatment. Normalized fluorescence values from drug-free cultures of C. albicans or A. fumigatus, stained with either CFDA or DiBAC, were compared with values obtained from cultures incubated with drug. The cell staining index was calculated from the formula normalized fluorescencetreated/normalized fluorescenceuntreated.
Microscopy.
Photomicrographs of cells were taken with a Zeiss Axiophot microscope equipped for both Nomarski optics and epifluorescence (with an FITC filter). Magnifications ranged from ×400 to ×2,000, and the film speed was either ASA 400 or ASA 1600. The exposure time for light micrographs was determined by the camera controller; for fluorescent micrographs, the exposure time was determined by the camera controller or manually, as noted where appropriate.
In vitro susceptibility.
A suspension of A. fumigatus MF5668 conidia was prepared as described above and was adjusted to an OD equal to that of the 0.5 McFarland standard by using a MicroScan turbidity meter. A concentration of approximately 1 × 106 to 5 × 106 conidia/ml was verified by counting with a hemocytometer. Susceptibility testing was performed by NCCLS protocol M38-P (32).
Cell counting.
Photomicrograph slides were scanned, and two-panel images, consisting of identical light and fluorescent micrographs of the same field, were printed at 600 pixels/in. The background color was subtracted from the fluorescent images so that only the green color of the fluorescent cells was visible; these panels were printed on transparencies and laid over the corresponding Nomarski image to ensure that the cells were appropriately identified as fluorescent or nonfluorescent. Septa between cells were identified on the basis of a published description (22). Cells were scored as apical (tip), subapical, or subapical branching. Cells whose boundaries were outside the field were not included in the totals. A single person performed all counting to reduce potential differences in the definitions of septa and cell position.
RESULTS
We sought to better understand the physiological effects of caspofungin treatment using CFDA and DiBAC, which are fluorescent indicators of cell viability (28). First, C. albicans strain MY1055 was grown in buffered RPMI 1640 medium and challenged with caspofungin to determine the appropriate conditions and timing for cell killing. Exposure of cells in the early logarithmic phase to 0.25 μg of caspofungin per ml reduced the number of CFU by 2 log10 in 15 h (data not shown). Cultures grown under these conditions were incubated with 0.15 μg of AMB per ml, 0.2 μg of caspofungin per ml, or 2.5 μg of FLC per ml or without drug. The cells were stained with either CFDA or DiBAC and were examined microscopically (Fig. 1). Nearly all cells from drug-free cultures stained with CFDA but not with DiBAC. Conversely, very few cells from AMB- or caspofungin-treated cultures stained with CFDA, while nearly all cells stained with DiBAC. C. albicans cells incubated with FLC showed a unique staining pattern; CFDA fluorescence was uniform but less intense than that seen for untreated cells, and only a few FLC-treated cells stained faintly with DiBAC.
FIG. 1.
Photomicrographs of untreated and drug-treated C. albicans stained with the fluorescent dyes CFDA and DiBAC. C. albicans cells from an untreated culture or from cultures treated with AMB (0.15 μg/ml), caspofungin (0.2 μg/ml), or FLC (2.5 μg/ml) were harvested after 15 h, stained with the dye CFDA, which stains viable cells, or the dye DiBAC, which stains nonviable cells, and photographed at ×1,000 magnification. Exposure times for epifluorescence of untreated samples stained with DiBAC, AMB-treated samples stained with CFDA, FLC-treated samples stained with CFDA, and FLC-treated samples stained with DiBAC were 20 s; all others were less than 1 s (as determined by the camera controller). Left panels, Nomarski optics; right panels, epifluorescence with an FITC filter set.
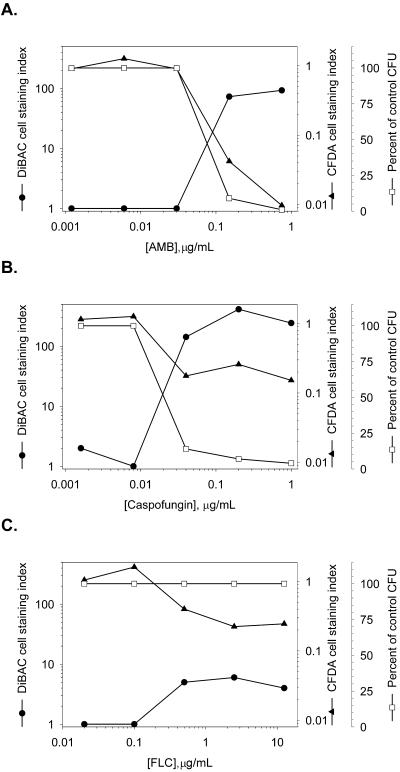
The effect of AMB, caspofungin, or FLC on dye staining of live or dead cells was quantified by fluorometry. Because fluorescence intensity is a function of both the physiological state of the cells and the culture density, we normalized each signal to the cell number determined by counting the cells with a hemocytometer. Titrations were performed with AMB, FLC, or caspofungin, and cells from drug-treated cultures were either stained with CFDA or DiBAC or spread onto agar plates to quantify the CFU (Fig. 2). At AMB concentrations ≥0.15 μg/ml, the number of CFU was reduced and there was a decrease in the amount of CFDA fluorescence per cell and a nearly 70-fold increase in the level of normalized DiBAC staining. Similarly, concentrations of caspofungin ≥0.04 μg/ml reduced the number of C. albicans CFU and caused a 4-fold decrease in the level of CFDA staining and a greater than 140-fold increase in the level of DiBAC staining compared to those for the controls. Concentrations of caspofungin or AMB that did not reduce the CFU counts also did not produce changes in staining with either dye. Exposure of C. albicans cells to FLC at concentrations ≥0.5 μg/ml caused a decrease in the level of CFDA staining, which we attribute to decreased fluorescence intensity in all cells rather than the all-or-none staining evident in caspofungin- and AMB-treated cultures (Fig. 1). There also was a <10-fold increase in the level of DiBAC staining of FLC-treated cells, but there was no reduction in the number of CFU at any concentration of FLC tested.
FIG. 2.
Dose-dependent changes in normalized CFDA or DiBAC fluorescence of C. albicans. Cultures were challenged with serial dilutions of AMB (A), caspofungin (B), or FLC (C). Aliquots of these cultures were stained with CFDA or DiBAC or were spread onto agar plates to determine the number of CFU. Fluorescence values were normalized per 106 cells (CFDA) or 107 cells (DiBAC), as described in Materials and Methods.
Having established that staining with CFDA and DiBAC reflects a loss of viability of caspofungin-treated C. albicans cells, we adapted this method to further investigate the activity of caspofungin against A. fumigatus. When A. fumigatus is grown in buffered RPMI 1640 medium, it forms tangled hyphal masses; aliquots removed by standard methods are not representative of the total culture. We modified the medium by adding the polyacrylate Junlon (5, 23) to a final concentration of 0.15% (wt/vol), which reduced mycelial masses and produced a culture of small, punctate clumps within a turbid suspension of individual germlings (Fig. 3A). The presence of Junlon did not have a deleterious effect on the amount of fungal growth, as evidenced by equivalent biomass (measured by determination of the total amount of protein) in cultures grown with or without the polyacrylate (data not shown). Samples of CFDA-stained cultures of A. fumigatus, grown in medium with or without Junlon, were serially diluted in the same medium, and the fluorescence was measured. The linear titration evident in the presence of Junlon (Fig. 3B) contrasted with the scattered, nonlinear fluorescence titration observed with cultures grown in RPMI 1640 medium alone. Individual hyphae of A. fumigatus had a similar appearance when grown in medium with or without Junlon (data not shown), and the addition of Junlon did not affect the susceptibility of A. fumigatus MF5668 to caspofungin, AMB, or ITC in standard liquid broth microdilution assays (Table 1).
FIG. 3.
In vitro growth of A. fumigatus in RPMI-Junlon. (A) Tubes containing RPMI 1640 medium (the two leftmost tubes [tubes 1 and 2]) or RPMI-Junlon (the two rightmost tubes [tubes 3 and 4]) were either inoculated with 105 A. fumigatus conidia (tubes 2 and 4) or left uninoculated (tubes 1 and 3) and were incubated for 15 h at 37°C. The tubes were swirled briefly prior to being photographed. (B) A. fumigatus conidia (105) were grown in either RPMI-Junlon (○) or RPMI 1640 medium alone (▴), incubated for 24 h at 37°C, and stained with CFDA. Stained samples were serially diluted twofold across a 96-well plate, and the fluorescence was read (excitation wavelength = 485 nm, emission wavelength = 538 nm).
TABLE 1.
In vitro susceptibility of A. fumigatus MF5668 in the absence or presence of Junlon
| Medium | MIC (μg/ml)a
|
|||||
|---|---|---|---|---|---|---|
| 24 h
|
48 h
|
|||||
| AMB | Caspofungin | ITC | AMB | Caspofungin | ITC | |
| RPMI 1640 | 0.06 | <0.03 | 0.125 | 0.25 | <0.03 | 0.25 |
| RPMI-Junlon | 0.06 | <0.03 | 0.125 | 0.125 | 0.06 | 0.25 |
The results shown are representative data from four independent assays. Values represent the lowest concentration of compound at which there was prominent growth inhibition relative to control. The MICs for isolate MF5668 are within the range of values obtained for clinical A. fumigatus isolates (A. M. Flattery, P. S. Hicks, A. Wilcox, and H. Rosen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 936, 2000).
We determined the effect of drug treatment on A. fumigatus hyphal morphology, cell staining, and CFU counts. Spores of A. fumigatus strain MF5668 inoculated into RPMI-Junlon and incubated with shaking at 37°C germinated and grew into small germlings within 14 h. Compound (either 0.15 μg of AMB per ml, 0.3 μg of caspofungin per ml, or 2.5 μg of ITC per ml) or vehicle was added to the actively growing cultures at this point. After 6 h of incubation with drug or vehicle, aliquots of the cultures were stained with either CFDA or DiBAC and examined microscopically. As with untreated C. albicans cultures, the vast majority of untreated A. fumigatus cells stained with CFDA but not with DiBAC (Fig. 4). In contrast, none of the AMB-treated A. fumigatus cells was stained by CFDA, but all AMB-treated A. fumigatus cells were stained by DiBAC. ITC treatment produced a forked or club-like morphology among a small number of apical cells, some of which failed to stain with CFDA or stained with DiBAC. Of the CFDA-positive ITC-treated cells, there was a trend toward decreased fluorescence of all cells, similar to the findings for FLC-treated C. albicans. Caspofungin-treated germlings had a high proportion of cells at hyphal tips and branch sites that did not stain with CFDA or that did stain with DiBAC (Fig. 4). Inspection of caspofungin-treated, DiBAC-stained hyphae at a higher magnification revealed plumes of stained material apparently outside of transparent cells at the tips (Fig. 5). This is most likely cellular phospholipid debris which was trapped in the viscous Junlon-containing medium after individual cells lysed and released their contents. The high prevalence of damaged cells at hyphal tips and the branch points of caspofungin-treated germlings was not reflected by a change in the number of CFU (Fig. 6). A. fumigatus germlings in RPMI-Junlon were exposed to 0.3 μg of caspofungin, AMB, or ITC per ml or vehicle and were monitored for the number of CFU during a 15-h incubation. AMB treatment produced an ∼2 log10 reduction in the number of A. fumigatus CFU within 3 h, and ITC or caspofungin treatment did not affect the number of CFU. Cultures grown in the absence of drug displayed obvious increases in hyphal mass without a commensurate increase in the number of CFU.
FIG.4.
Photomicrographs of untreated and drug-treated A. fumigatus stained with the fluorescent dyes CFDA and DiBAC. A. fumigatus germlings incubated for 6 h with 0.15 μg of AMB per ml, 0.3 μg of caspofungin per ml, or 2.5 μg of ITC per ml or without drug were stained with the dye CFDA, which stains viable cells, or the dye DiBAC, which stains nonviable cells, and photographed at ×400 magnification. Fluorescent micrograph exposure times for untreated samples stained with DiBAC, AMB-treated samples stained with CFDA, and ITC-treated samples stained with CFDA or DiBAC were 20 s; all others were less than 1 s (as determined by the camera controller). Left panels, Nomarski optics; right panels, epifluorescence with an FITC filter set.
FIG. 5.
High-magnification photomicrographs of caspofungin-treated, DiBAC-stained A. fumigatus. A. fumigatus germlings incubated for 6 h with 0.3 μg of caspofungin per ml were stained with DiBAC and photographed at ×2,000 magnification. Images from two separate germlings from the same culture are shown. Left panels, Nomarski optics; right panels, epifluorescence with an FITC filter set.
FIG. 6.
A. fumigatus killing curves. A. fumigatus conidia (105) were inoculated into RPMI-Junlon and incubated at 37°C for 14 h before 0.3 μg of drug per ml (AMB [▵], caspofungin [▾], or ITC [□]) or vehicle (○) was added. At 3-h intervals after drug addition, aliquots from each culture were spread onto agar plates for determination of the number of CFU.
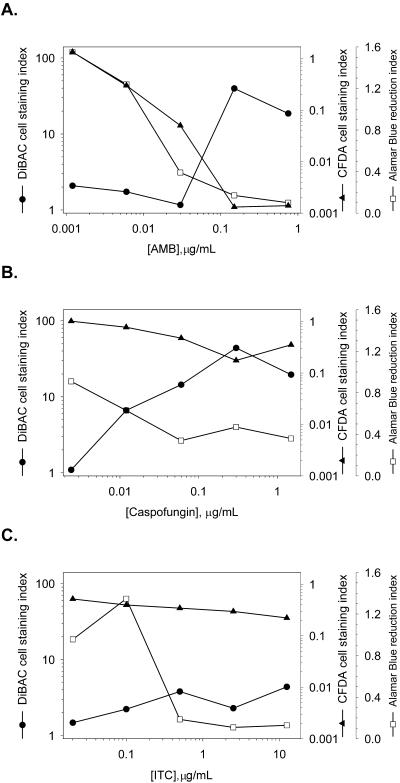
The dose dependence of drug-induced changes in A. fumigatus staining with CFDA or DiBAC was evaluated by titrating each compound and measuring the fluorescence quantitatively. To relate any changes in staining to cell growth and viability, parallel cultures were monitored for the reduction of Alamar Blue in the presence or the absence of drug. A change in the color of the Alamar Blue dye results from the production of reducing equivalents during fungal growth; there is little to no change in dye color if cells die or stop growing. AMB-treated A. fumigatus cells exhibited a threshold for increased DiBAC staining and decreased CFDA staining at AMB concentrations ≥0.15 μg/ml (Fig. 7). This threshold correlated with the reduction of Alamar Blue and was consistent with the staining patterns observed in photomicrographs (Fig. 4). Caspofungin-treated A. fumigatus cultures exhibited a continuum of normalized fluorescence changes and Alamar Blue reduction across a broad range of concentrations, with no clear threshold. A. fumigatus treated with 0.012 or 0.06 μg of caspofungin per ml displayed moderate DiBAC staining; the maximal response was at caspofungin concentrations ≥0.3 μg/ml. This correlated with a decreased level of Alamar Blue reduction. ITC at concentrations ≥0.5 μg/ml prevented Alamar Blue reduction, with little quantitative change in fluorescent dye staining. At these concentrations, ITC appears to stop the production of reducing equivalents by A. fumigatus while minimally inducing the physiological changes reflected by changes in CFDA or DiBAC staining.
FIG. 7.
Quantitative fluorescence of dye-stained A. fumigatus. A. fumigatus cultures were grown as described in the text and incubated with serial dilutions of AMB (A), caspofungin (B), or ITC (C) for 6 h. Aliquots of these cultures were stained with CFDA or DiBAC; parallel titrations were performed with cultures containing 10% (vol/vol) Alamar Blue. The values for CFDA and DiBAC staining were normalized to the total amount of protein, and the fold change relative to the amount for the untreated control (staining index) was determined as described in Materials and Methods.
The preponderance of staining changes among cells at the tips and branch points of caspofungin-treated A. fumigatus germlings prompted an analysis of the effects of caspofungin as a function of cell position. A. fumigatus cells in photomicrographs were categorized as either apical, subapical, or subapical branched (Fig. 8) and were scored as fluorescent or nonfluorescent. As seen in Fig. 4 and Table 2, AMB-treated A. fumigatus cells failed to stain with CFDA and stained with DiBAC, regardless of their position in the germ tube. Among caspofungin-treated germlings, only 12% of apical cells stained with CFDA, and 72% stained with DiBAC, while 39% of subapical branching cells stained with CFDA and 56% stained with DiBAC (Table 2). For subapical cells within caspofungin-treated germlings, 76% stained with CFDA and 10% stained with DiBAC. ITC treatment led to a staining pattern that was intermediate in its cell specificity; there was a modest preference for apical and subapical branching cells that was not as pronounced as the selective staining pattern seen with caspofungin-treated germlings.
FIG. 8.
Classification scheme for cells within A. fumigatus germlings. An A. fumigatus culture was treated with 0.3 μg of caspofungin per ml, stained with the viable dye CFDA, and photographed at ×1,600 magnification. Left panel, Nomarski optics; right panel, epifluorescence with an FITC filter set plus bright-field illumination. Septa (indicated by arrows) were identified as described in the text, and the cells were classified as apical (e.g., cells 1 and 6), subapical (e.g., cells 3 and 5), or subapical branching (e.g., cells 2 and 4).
TABLE 2.
Drug-induced staining changes in A. fumigatus as a function of cell typea
| Stain | Treatment | % Fluorescent cells identified as:
|
||
|---|---|---|---|---|
| Apical | Subapical branching | Subapical | ||
| CFDA | None | 100 (18/18)b | 100 (22/22) | 96 (107/112) |
| AMBc | 0 (0/5) | 0 (0/7) | 0 (0/6) | |
| Caspofungin | 12 (6/49) | 39 (25/64) | 76 (52/68) | |
| ITC | 44 (28/64) | 76 (38/50) | 86 (107/124) | |
| DiBAC | Noned | 0 (0/28) | 3 (1/30) | 1 (2/139) |
| AMBc | 100 (15/15) | 100 (27/27) | 100 (10/10) | |
| Caspofungin | 72 (72/100) | 56 (51/91) | 10 (10/97) | |
| ITC | 61 (51/83) | 37 (15/41) | 26 (44/171) | |
Photomicrographs (Nomarski and epifluorescence images) of untreated A. fumigatus germlings or germlings treated with 0.15 μg of AMB per ml, 0.3 μg of caspofungin per ml, or 2.5 μg of ITC per ml were scanned. Fluorescent cells stained with either dye were counted as described in Materials and Methods.
Values in parenthesis indicate the number of fluorescent cells/total number of cells counted. Results represent the sum of three independent experiments unless noted otherwise.
Results from one experiment.
Sum of five independent experiments.
We explored the effect of extended incubation in the presence of each antifungal drug on dye staining. Aliquots removed at 6, 24, 48, and 72 h after drug addition from cultures grown in RPMI-Junlon were stained and evaluated by microscopy, and parallel cultures were grown in the presence of Alamar Blue. There was substantial growth in the untreated culture during the course of the experiment (data not shown), and the CFDA-stained hyphae fluoresced strongly at all time points. There was a slight increase in DiBAC staining of untreated germlings with incubation for ≥24 h, although this was primarily due to staining of conidia and conidium-bearing structures (Fig. 9A). AMB at concentrations ≥0.27 μg/ml caused changes in staining by CFDA and DiBAC, regardless of the cell position within the germlings, over the entire 72-h time course (data not shown). Of interest, 0.09 μg of AMB per ml had antifungal activity at 6 and 24 h, but there was significant growth by 48 h. This was confirmed both by staining with CFDA and DiBAC and by Alamar Blue reduction. At all time points, caspofungin preferentially affected actively growing cells at the tips and branch points of A. fumigatus hyphae, producing staining changes in roughly 75% of apical cells (Fig. 9 and data not shown). The extent of hyphal branching became more pronounced with prolonged incubation, but the proportion of fluorescent apical and subapical branching cells was consistent at all time points. With as much as 72 h of incubation with caspofungin at concentrations ≥0.06 μg/ml, there was no evidence of hyphae with the slender, tapered morphology of untreated A. fumigatus germlings. Exposure to ITC caused only subtle changes in the CFDA and DiBAC staining patterns at all concentrations tested, although there was growth inhibition and no detectable Alamar Blue reduction at ITC concentrations ≥0.5 μg/ml (data not shown).
FIG. 9.
Effect of extended drug treatment on A. fumigatus fluorescent dye staining. (A) Untreated, DiBAC-stained germlings harvested 24 h after vehicle addition. Magnification, ×400. Arrows indicate conidiophores. Caspofungin-treated germlings (0.3 μg/ml) harvested 72 h after drug addition were stained with CFDA (B) or DiBAC (C), and photographed at ×800 magnification. In panels B and C, arrows with single tails indicate lysed apical cells and arrows with double tails indicate lysed conidiophores. The fluorescent micrograph in panel A required a 20-s exposure. Left panels, Nomarski optics; right panels, epifluorescence with an FITC filter set.
Morphological changes associated with sporulation were observed with prolonged incubation in both drug-free and caspofungin-treated cultures. This phenomenon is distinct from microcycle conidiation (direct production of conidiophores without prior mycelial formation), but like microcycle conidiation, it was most likely a consequence of growth at 37°C in shake flasks (3, 10). Without drug, abundant conidiophores formed from submerged hyphae within 24 h, and their morphology resembled that of structures produced by aerial mycelia. In contrast, aberrant conidiophores formed by caspofungin-treated A. fumigatus appeared only after 72 h, they were much less prevalent, and they appeared transparent and flattened (Fig. 9). At least one-half failed to stain with CFDA or stained with DiBAC, and none appeared to produce any mature conidia.
DISCUSSION
The effects of antifungal compounds on fungal pathogens are frequently tested by spreading samples from either treated cultures or organs from infected animals on agar plates and enumerating the CFU. However, for A. fumigatus, the filamentous nature of growth in both liquid culture and infected tissues makes it difficult to assess activity based on the number of CFU. Increases in cell mass are not usually reflected by increases in the number of CFU (29), and a CFU by definition reflects the viability only of the entire germling, not of individual cells within the germling. Because of the limitations of CFU measurements for A. fumigatus, alternative methods have been developed which utilize dye staining to assess various aspects of cell viability, most notably, membrane potential and intracellular enzymatic activity. Examples of these stains include tetrazolium dyes (14, 15) and the halogenated dye FUN-1 (27). Another dye, propidium iodide, has been used in flow cytometric analysis of C. albicans and C. neoformans (21), but its use with A. fumigatus to date has been limited to conidia (30).
A previous report (28) described the use of CFDA and DiBAC to characterize the response of C. albicans to AMB. Among other things, these dyes have been used to study the membrane potentials of mammalian cells (7, 12) and the viabilities of both bacteria (24) and S. cerevisiae (16). By using at least two different dyes, which provide information about independent aspects of cell physiology, drugs with distinct mechanisms of action can be compared. It is important to relate preliminary dye-staining results to other viability measurements, including CFU quantitation or Alamar Blue reduction, to ensure that the staining changes correspond to true changes in cell vitality.
The mycelial masses which form as A. fumigatus grows in liquid culture confounded our ability to withdraw representative samples or to focus on a field of cells under the microscope. We found that the addition of the polyacrylate Junlon promoted a more homogeneous culture of A. fumigatus in shaking flasks. After drug treatment and dye staining, a linear titration was obtained when germlings grown in RPMI-Junlon were diluted serially and assayed for fluorescence. Junlon has been used to force dispersed growth of other organisms, including Streptomyces spp. (23) and Aspergillus niger (5, 39). Additionally, by selecting germlings trapped in the Junlon layer, we focused on a form of A. fumigatus which not only fit entirely within a single microscope field but which was also representative of the total culture. The ability to photograph entire germlings and discriminate individual cells within these germlings was especially useful for assessing the preferential lysis of growing A. fumigatus cells by caspofungin.
The abundance of 1,3-β-d-glucan in cell walls formed during different stages of the A. fumigatus life cycle is not well characterized. During vegetative growth, the focus of new cell wall synthesis is the hyphal apex (4, 9, 37), and inhibition of 1,3-β-d-glucan synthesis has profound effects on cell wall structure in A. fumigatus (26). As predicted from work with A. nidulans mutants, whose altered cell wall composition led to hyphal “balloons” which lysed without osmotic protection (40), we found that cells at the hyphal tips and branch points were more susceptible to lysis than subapical cells with mature cell walls. Even with prolonged incubation (up to 72 h), the focus of caspofungin killing was on apical and subapical branching cells. As aberrant, conidiophore-like structures formed in the presence of caspofungin, their staining pattern with CFDA or DiBAC was also consistent with cell death. Therefore, our results demonstrate that inhibition of 1,3-β-d-glucan synthesis during either germ tube or conidiophore formation can be a lethal event. Understanding the effect of glucan synthesis inhibition as spores germinate, which is another key developmental step for A. fumigatus, will require further study.
Because filamentous fungi grow by apical extension, continued killing of cells at the hyphal tips would be expected to have a profound impact on the growth of the organism in vivo. Petraitiene et al. (33) reported that caspofungin treatment improved survival compared to that for the untreated controls in a rabbit model of pulmonary aspergillosis. This improvement in survival was at least as good as that seen with AMB therapy. Caspofungin treatment reduced several clinical measures of disease, including total lung weight, pulmonary infarcts, and pulmonary injury (determined by computed tomography), despite an increase in the number of CFU. In a recent report, caspofungin treatment yielded a reduced fungal burden (assessed by quantitative PCR) compared to that in untreated animals, and the reduction in the fungal burden was comparable to that determined for an AMB-treated group (11).
The drugs used in these studies (AMB, FLC, ITC, and caspofungin) have been categorized as either fungicidal or fungistatic. AMB is accepted as fungicidal against both C. albicans and A. fumigatus, based on the reductions in the number of CFU. Conversely, azoles such as FLC and ITC are considered fungistatic against C. albicans and A. fumigatus, respectively. It is interesting that both FLC- and ITC-treated cells exhibited weak but widespread staining with CFDA in our study. Perhaps azole-mediated ergosterol inhibition causes increased permeability of the cell membranes, such that azole-treated cells only partially retain free carboxyfluorescein following esterase cleavage of CFDA. Caspofungin has been shown to be fungicidal against C. albicans (2, 6), and the fluorescence patterns observed in cells stained with CFDA and DiBAC correlate with decreases in the number of CFU in a manner comparable to that seen with AMB-treated cells. The activity of caspofungin against A. fumigatus is more difficult to categorize. While there was no reduction in the number of CFU from standard killing curve measurements, we did see changes in the patterns of staining by dyes that stain viable and nonviable cells analogous to those observed with caspofungin-treated C. albicans. Plumes of DiBAC-stained debris at hyphal tips suggest caspofungin caused lysis of the actively growing A. fumigatus cells. Whether lysis occurs in vivo remains to be determined. Work with persistently neutropenic mice illustrated that caspofungin prevents significant mortality in A. fumigatus-infected animals, even for up to 21 days after therapy is withdrawn (2). If caspofungin is strictly fungistatic against A. fumigatus, a reemergence of the infection should lead to mortality in the face of continued immunosuppression. Perhaps significant injury to cells at the hyphal tips renders the fungus unable to invade tissue beyond a focus of infection. As subapical cells within the mycelium form branches, their requirement for de novo synthesis of 1,3-β-d-glucan may render them susceptible to lysis by caspofungin. The inability of A. fumigatus to sustain polarized growth in the presence of multiple doses of caspofungin could lead to significant fungal cell death in tissues.
Acknowledgments
We thank Jill Baimel for film development and creation of graphics; John Allocco, Jennifer Anderson, Lissa Cherry, Larry Koupal, Maria Meinz, and Jan Onishi for assistance with dye-staining experiments; and Mark Rosenbach for suggestions regarding Junlon.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abruzzo, G. K., C. J. Gill, A. M. Flattery, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, and H. Rosen. 2000. Efficacy of the echinocandin caspofungin against disseminated aspergillosis and candidiasis in cyclophosphamide-induced immunosuppressed mice. Antimicrob. Agents Chemother. 44:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, J. G., and J. E. Smith. 1971. The production of conidiophores and conidia by newly germinated conidia of Aspergillus niger (microcycle conidiation). J. Gen. Microbiol. 69:185-197. [DOI] [PubMed] [Google Scholar]
- 4.Archer, D. B. 1977. Chitin biosynthesis in protoplasts and subcellular fractions of Aspergillus fumigatus. Biochem. J. 164:653-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer, D. B., D. A. MacKenzie, and M. J. Ridout. 1995. Heterologous protein secretion by Aspergillus niger growing in submerged culture as dispersed or aggregated mycelia. Appl. Microbiol. Biotechnol. 44:157-160. [DOI] [PubMed] [Google Scholar]
- 6.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bashford, C. L., G. M. Alder, M. A. Gray, K. J. Micklem, C. C. Taylor, P. J. Turek, and C. A. Pasternak. 1985. Oxonol dyes as monitors of membrane potential: the effect of viruses and toxins on the plasma membrane potential of animal cells in monolayer culture and in suspension. J. Cell. Physiol. 123:326-336. [DOI] [PubMed] [Google Scholar]
- 8.Bashford, C. L., B. Chance, J. C. Smith, and T. Yoshida. 1979. The behavior of oxonol dyes in phospholipid dispersions. Biophys. J. 25:63-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauvais, A., J. M. Bruneau, P. C. Mol, M. J. Buitrago, R. Legrand, and J. P. Latge. 2001. Glucan synthase complex of Aspergillus fumigatus. J. Bacteriol. 183:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobbitt, T. F., and C. M. Douglas. 1982. Microcycle conidiation by regenerating protoplasts of Aspergillus awamori. Exp. Mycol. 6:307-311. [Google Scholar]
- 11.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bräuner, T., D. F. Hülser, and R. J. Strasser. 1984. Comparative measurements of membrane potentials with microelectrodes and voltage-sensitive dyes. Biochim. Biophys. Acta 771:208-216. [DOI] [PubMed] [Google Scholar]
- 13.Cassone, A., R. E. Mason, and D. Kerridge. 1981. Lysis of growing yeast-form cells of Candida albicans by echinocandin: a cytological study. Sabouraudia 19:97-110. [PubMed] [Google Scholar]
- 14.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2001. The interaction of human monocytes, monocyte-derived macrophages, and polymorphonuclear neutrophils with caspofungin (MK-0991), an echinocandin, for antifungal activity against Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 39:99-103. [DOI] [PubMed] [Google Scholar]
- 15.Chiou, C. C., N. Mavrogiorgos, E. Tillem, R. Hector, and T. J. Walsh. 2001. Synergy, pharmacodynamics, and time-sequenced ultrastructural changes of the interaction between nikkomycin Z and the echinocandin FK463 against Aspergillus fumigatus. Antimicrob. Agents Chemother. 45:3310-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinsdale, M. G., and D. Lloyd. 1995. Yeast vitality during cider fermentation: two approaches to the measurement of membrane potential. J. Inst. Brew. 101:453-458. [Google Scholar]
- 17.Douglas, C. M., J. A. D'Ippolito, G. J. Shei, M. Meinz, J. Onishi, J. A. Marrinan, W. Li, G. K. Abruzzo, A. Flattery, K. Bartizal, A. Mitchell, and M. B. Kurtz. 1997. Identification of the FKS1 gene of Candida albicans as the essential target of 1,3-β-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 41:2471-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas, C. M., F. Foor, J. A. Marrinan, N. Morin, J. B. Nielsen, A. M. Dahl, P. Mazur, W. Baginsky, W. Li, M. el-Sherbeini, et al. 1994. The Saccharomyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1,3-β-d-glucan synthase. Proc. Natl. Acad. Sci. USA 91:12907-12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst, E. J., M. E. Klepser, M. E. Ernst, S. A. Messer, and M. A. Pfaller. 1999. In vitro pharmacodynamic properties of MK-0991 determined by time-kill methods. Diagn. Microbiol. Infect. Dis. 33:75-80. [DOI] [PubMed] [Google Scholar]
- 20.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green, L., B. Petersen, L. Steimel, P. Haeber, and W. Current. 1994. Rapid determination of antifungal activity by flow cytometry. J. Clin. Microbiol. 32:1088-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gull, K. 1978. Form and function of septa in filamentous fungi, p. 78-93. In J. E. Smith and D. R. Berry (ed.), The filamentous fungi, vol. 3. John Wiley & Sons, Inc., New York, N.Y.
- 23.Hobbs, G., C. M. Frazer, D. C. J. Gardner, J. A. Cullum, and S. G. Oliver. 1989. Dispersed growth of Streptomyces in liquid culture. Appl. Microbiol. Biotechnol. 31:272-277. [Google Scholar]
- 24.Jepras, R. I., J. Carter, S. C. Pearson, F. E. Paul, and M. J. Wilkinson. 1995. Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl. Environ. Microbiol. 61:2696-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly, R., E. Register, M. J. Hsu, M. Kurtz, and J. Nielsen. 1996. Isolation of a gene involved in 1,3-β-glucan synthesis in Aspergillus nidulans and purification of the corresponding protein. J. Bacteriol. 178:4381-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lass-Flörl, C., M. Nagl, C. Speth, H. Ulmer, M. P. Dierich, and R. Würzner. 2001. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob. Agents Chemother. 45:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao, R. S., R. P. Rennie, and J. A. Talbot. 1999. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob. Agents Chemother. 43:1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manavathu, E. K., J. L. Cutright, and P. H. Chandrasekar. 1998. Organism-dependent fungicidal activities of azoles. Antimicrob. Agents Chemother. 42:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marr, K. A., M. Koudadoust, M. Black, and S. A. Balajee. 2001. Early events in macrophage killing of Aspergillus fumigatus conidia: new flow cytometric viability assay. Clin. Diagn. Lab. Immunol. 8:1240-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Momany, M., P. J. Westfall, and G. Abramowsky. 1999. Aspergillus nidulans swo mutants show defects in polarity establishment, polarity maintenance and hyphal morphogenesis. Genetics 151:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. NCCLS document M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 33.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petraitis, V., R. Petraitiene, A. H. Groll, A. Bell, D. P. Callender, T. Sein, R. L. Schaufele, C. L. McMillian, J. Bacher, and T. J. Walsh. 1998. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob. Agents Chemother. 42:2898-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251-255. [DOI] [PubMed] [Google Scholar]
- 36.Pringle, J. R., R. A. Preston, A. E. Adams, T. Stearns, D. G. Drubin, B. K. Haarer, and E. W. Jones. 1989. Fluorescence microscopy methods for yeast. Methods Cell Biol. 31:357-435. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz-Herrera, J. 1992. Fungal cell wall: structure, synthesis, and assembly. CRC Press, Inc., Boca Raton, Fla.
- 38.Thompson, J. R., C. M. Douglas, W. Li, C. K. Jue, B. Pramanik, X. Yuan, T. H. Rude, D. L. Toffaletti, J. R. Perfect, and M. Kurtz. 1999. A glucan synthase FKS1 homolog in Cryptococcus neoformans is single copy and encodes an essential function. J. Bacteriol. 181:444-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinci, A. P. J. 1983. Effect of Junlon on morphology of Aspergillus niger and its use in making turbidity measurements of fungal growth. Trans. Br. Mycol. Soc. 81:408-412. [Google Scholar]
- 40.Valentine, B. P., and B. W. Bainbridge. 1978. The relevance of a study of a temperature-sensitive ballooning mutant of Aspergillus nidulans defective in mannose metabolism to our understanding of mannose as a wall component and carbon/energy source. J. Gen. Microbiol. 109:155-168. [Google Scholar]