Abstract
Background
Previous literature lacks summative information on the mental health benefits achieved from different forms of walking.
Objective
The aim of this study was to assess the effectiveness of different forms of walking in reducing symptoms of depression and anxiety.
Methods
This was a systematic review and meta-analysis of randomized controlled trials (RCTs) assessing the effects of walking on depressive and anxiety symptoms. MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), Embase, PsycINFO, Allied and Complementary Medicine Database (AMED), CINAHL, and Web of Science were searched on April 5, 2022. Two authors independently screened the studies and extracted the data. Random-effects meta-analysis was used to synthesize the data. Results were summarized as standardized mean differences (SMDs) with 95% CIs in forest plots. The risk of bias was assessed by using the Cochrane Risk of Bias tool.
Results
This review included 75 RCTs with 8636 participants; 68 studies reported depressive symptoms, 39 reported anxiety symptoms, and 32 reported both as the outcomes. One study reported the results for adolescents and was not included in the meta-analysis. The pooled results for adults indicated that walking could significantly reduce depressive symptoms (RCTs: n=44; SMD −0.591, 95% CI −0.778 to −0.403; I2=84.8%; τ2=0.3008; P<.001) and anxiety symptoms (RCTs: n=26; SMD −0.446, 95% CI −0.628 to −0.265; I2=81.1%; τ2=0.1530; P<.001) when compared with the inactive controls. Walking could significantly reduce depressive or anxiety symptoms in most subgroups, including different walking frequency, duration, location (indoor or outdoor), and format (group or individual) subgroups (all P values were <.05). Adult participants who were depressed (RCTs: n=5; SMD −1.863, 95% CI −2.764 to −0.962; I2=86.4%; τ2=0.8929) and those who were not depressed (RCTs: n=39; SMD −0.442, 95% CI −0.604 to −0.280; I2=77.5%; τ2=0.1742) could benefit from walking effects on their depressive symptoms, and participants who were depressed could benefit more (P=.002). In addition, there was no significant difference between walking and active controls in reducing depressive symptoms (RCTs: n=17; SMD −0.126, 95% CI −0.343 to 0.092; I2=58%; τ2=0.1058; P=.26) and anxiety symptoms (14 RCTs, SMD −0.053, 95% CI −0.311 to 0.206, I2=67.7%, τ2=0.1421; P=.69).
Conclusions
Various forms of walking can be effective in reducing symptoms of depression and anxiety, and the effects of walking are comparable to active controls. Walking can be adopted as an evidence-based intervention for reducing depression and anxiety. More evidence on the effect of low-intensity walking is needed in the future.
Introduction
Depression and anxiety are common mental disorders, with the global prevalence of depression in 2023 being 5% and that of anxiety disorders being 4% [1-3]. About 18.4% and 33.7% of people experience depression and anxiety disorders, respectively, at some time in their lives [4-6]. Globally, the age-standardized, disability-adjusted life-year rates for depressive disorders and anxiety disorders were 585 and 359 per 100,000 population, respectively, in 2019, which accounted for the two largest proportions of mental disorder disability-adjusted life-years [7]. In addition, there are many more people experiencing subthreshold depression and anxiety [8,9], especially during the COVID-19 pandemic. Interventions are available to treat depression and anxiety or reduce their symptoms, including antidepressants, psychotherapy, and physical exercise [10]. Walking, as one type of physical exercise, is considered safe, convenient, and noninvasive; it has low or no costs and does not require any special equipment or training, unlike some other forms of exercise [11]. It is a good intervention candidate that can be easily implemented, particularly for people with subthreshold depression and anxiety or patients who may not have access to or prefer not to take medication or therapy.
In recent years, more and more studies on walking have been conducted and have attracted attention from the public [12-14]. Prior systematic reviews found walking to have benefits for various aspects of health, such as improvements in cardiorespiratory health [15], blood pressure [16], cholesterol levels [17], chronic pain [18], and weight loss [19], as well as reductions in the risk for diabetes [20] and all-cause mortality [21].
Apart from its benefits for physical health, previous systematic reviews and meta-analyses were conducted to measure the effect of walking on depressive symptoms. A previous meta-analysis of 8 randomized controlled trials (RCTs) with a total of 341 participants who were depressed found that walking interventions had a significant, large, standardized mean difference (SMD) of −0.86 for depressive symptoms in adult participants [22]. In 2015, another meta-analysis with a total of 110 adult participants indicated a significant benefit of outdoor group walking for depressive symptoms, with an SMD of −0.67 [23]. Other meta-analyses only focused on a specific group of people or one form of walking, such as people with postpartum depression and nature walking [24,25]. These previous studies only included a small number of RCTs with a small total number of participants or focused on a specific population or form of walking. More RCTs on walking have been published in recent years but have not been included in any systematic reviews; a review of these RCTs, which provide more robust evidence, would allow for a more comprehensive understanding of the effects of walking. In addition, previous reviews did not distinguish between different types of control groups. More importantly, previous meta-analyses lacked detailed information on other forms of walking that can provide mental health benefits, such as indoor and outdoor walking, individual and group walking, walking facilitated by equipment (eg, pedometers), and nonfacilitated walking. Furthermore, it remains unclear whether specific factors of walking, such as various walking characteristics (eg, duration, intensity, frequency, and pace) or walking with or without instructions, affect the results of walking interventions. Understanding the information on different forms of walking can help with informing health care professionals about how to promote walking in a very specific way, supporting mental health services in community and clinical settings, and informing public health policies on walking promotion [26]. This systematic review and meta-analysis was undertaken to assess the effects of walking and its different forms on reducing symptoms of depression and anxiety via comparisons with inactive controls and active controls, using the latest available evidence.
Methods
This review was registered with PROSPERO (International Prospective Register of Systematic Reviews; CRD42021247983) and was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [27].
Literature Search
Systematic searches were restricted to English-language articles in electronic databases that were published from the date that the database was established to April 5, 2022. The seven databases searched included MEDLINE (PubMed), Cochrane Central Register of Controlled Trials (CENTRAL), Embase, PsycINFO, Allied and Complementary Medicine Database (AMED), CINAHL, and Web of Science. The strategies used for the search included the following: (walk* OR pedometer* OR step count) AND (depress* OR anxiety OR anxio*) AND (randomized controlled trial OR controlled clinical trial). The full search strategies can be found in Table S1 in Multimedia Appendix 1. Articles from relevant systematic reviews and meta-analyses were included. Backward and forward reference searching were also performed on the articles identified from relevant systematic reviews and meta-analyses.
Four authors (ZX, XZ, HD, and PMHC) were involved in the article screening, using Covidence (Veritas Health Innovation). Two authors independently performed the screening of titles and abstracts after Covidence automatically removed the duplicate articles. Two authors then reviewed the full texts of short-listed articles. With regard to articles for which full texts were not accessible on the internet, we sent emails to these articles’ authors to request full texts directly. Another author (DZ) was involved in the discussions to resolve any discrepancies in the title and abstract screening and the full-text review.
Eligibility Criteria
The detailed inclusion and exclusion criteria are summarized in Table 1.
Table 1. Inclusion and exclusion criteria of this systematic review, which assessed the effect of walking on depression and anxiety.
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | No restriction on the population | None |
| Intervention | Any kind of walking intervention | A mixed intervention, such as combining walking with other physical activities, supplements, or psychosocial intervention, and 1-bout or 1-day walking |
| Control | No intervention or interventions other than walking | Control group containing walking |
| Outcome | Any scale measuring symptoms of depression and anxiety at baseline and after intervention | Scales measuring overall psychological well-being instead of depressive and anxiety symptoms |
| Study design | Randomized controlled trial | Interventional study without a control group, case report, cross-sectional study, cohort study, case-control study, qualitative study, and review |
| Article type | Research paper | Conference paper or letter |
| Language | English | Not English |
| Other | Full text available | Full text not available |
Data Extraction
Three authors (ZX, XZ, and HD) were involved in the data extraction. Two authors independently extracted data from all eligible articles. The extracted data included the title, first author, year of publication, country, participant characteristics (age, gender, and health condition), details of the intervention (eg, intervention duration and the intensity, frequency, and duration of each walk), and outcomes (symptoms of depression and anxiety measured by scales).
The Cochrane Collaboration Recommendations assessment tool was used to evaluate the risk of bias for the included studies [28]. Due to the nature of the walking interventions, the participants could not be blinded to the group allocation. Therefore, the item “blinding of participants and personnel” was not included in this study. The level of the risk of bias for each item and the overall risk of bias were classified as low, unclear, and high. If the risk of bias level for all items was classified as low, the overall risk of bias for the study was defined as low. If the risk of bias level for at least one item was rated as high, the overall risk of bias was defined as high. If the risk of bias level for at least one item was unclear and no item was rated as high risk, the overall risk of bias was defined as unclear [29]. For any discrepancies in data extraction and the assessed risk of bias between the aforementioned authors, another author (DZ) was referred to and made a decision after discussion.
Statistical Analysis
The effect size SMDs for depressive and anxiety symptoms were initially calculated from the mean differences and SDs between the baseline and postintervention data of each study. If the SD of the mean difference was not available in a study, it was calculated from SEs and 95% CIs, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [28]. When the SD could not be calculated, the median correlation coefficients between baseline and postintervention scores, which were calculated by using the median correlations of studies without missing data, were used to estimate the missing SDs for scales of depression and anxiety [28].
The nonwalking comparators were categorized as inactive controls, active controls, and other controls. Inactive controls included no intervention, waitlists, usual care that did not involve treating depression or anxiety, brief instructions on physical activity, and placebo meetings, in accordance with a previous meta-analysis and a decision framework about control conditions for RCTs in psychiatry [30,31]. Active controls were defined as evidence-based interventions that have been shown to have positive effects on depression and anxiety, such as moderate-intensity exercise (eg, strengthening exercise, resistance exercise, swimming, cycling, jogging, Pilates, and stabilization exercise), yoga [32], tai chi [33], meditation [34], cognitive behavioral therapy [35], stress management training [36], art therapy [37], and social interaction [38]. Other controls included light exercise (eg, low-intensity stretching or relaxation exercises), nutrition-related control, and regular education, which lack enough evidence of their effects on depression and anxiety. The primary analysis was the comparison between walking and inactive controls, and the secondary analysis compared walking with active controls and other controls.
The meta-analysis was performed by using the random-effects model [39]. The SMD was used as the outcome to pool the results of the different scales. The SMDs of each study, their 95% CIs, and the pooled results were also presented in forest plots. I² and τ² were reported to indicate the heterogeneity of studies [40]. Small-study effects, which mainly include publication bias, were assessed by using funnel plots and the Egger test [41,42]. A subgroup analysis was conducted according to the different forms or characteristics of the walking interventions. The definition of each subgroup is summarized in Table S2 in Multimedia Appendix 1. The forms of walking with a P value of <.05 in the subgroup analysis were entered into the multivariate meta-regression. The significance level of the between-group differences was computed, and a 2-tailed P value of <.05 was considered statistically significant. All statistical analyses were performed by using Stata (version 16.0; StataCorp LLC).
Ethical Considerations
This was a systematic review and meta-analysis and thus did not require ethical approval. This work was conducted following the World Medical Association's Declaration of Helsinki.
Results
Study Selection
Figure 1 shows the flow diagram of the study selection process. A total of 23,817 records were identified from the 7 databases, and 1853 records were identified from relevant systematic reviews and via backward and forward reference searching. After removing 7820 duplicate records, 17,850 records were screened based on the titles and abstracts, and 940 of them were assessed for eligibility by reviewing the full texts. Finally, 75 RCTs were included in the systematic review.
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of this systematic review, which assessed the effects of walking and its different forms on reducing symptoms of depression and anxiety. The flow diagram depicts the identification of studies via databases and other sources. AMED: Allied and Complementary Medicine Database.
Study Characteristics
The characteristics of the included studies are shown in Table S3 in Multimedia Appendix 1 [43-117]. The 75 RCTs had a total of 8636 participants. In one study, participants’ mean age was 16.8 years, and the mean ages of the participants in the remaining studies ranged from 30.4 to 84.7 years. The intervention duration ranged from 10 days to 18 months, with 44 (59%) studies having 3 to 6 months of intervention. The participants in 10 (13%) studies were depressed at baseline, in accordance with these studies’ inclusion criteria; the participants in 5 studies were diagnosed with depression, and the participants in 5 studies screened positive for depressive symptoms based on scales. No study was on patients diagnosed with anxiety. The sample sizes ranged from 17 to 1023 participants, with 25 (33%) studies having a sample size of ≥100. A total of 68 studies reported depressive symptoms as the outcome, 39 studies reported anxiety symptoms, and 32 studies reported both.
The risk of bias in each study is shown in Table S4 in Multimedia Appendix 1 [43-117] and is summarized in Figure 2. The risk of bias was low for random sequence generation in 39 (52%) studies, allocation concealment in 18 (24%) studies, blinding of the outcome assessment in 24 (32%) studies, incomplete outcome data in 60 (80%) studies, selective reporting in 74 (99%) studies, and other bias in 75 (100%) studies. Overall, 6 (8%) studies had a low risk of bias, 52 (69%) studies had an unclear risk of bias, and 17 (23%) had a high risk of bias.
Figure 2. Summary of risk of bias for studies assessing the effect of walking on symptoms of depression and anxiety. Risk of bias was evaluated by using the Cochrane Risk of Bias tool.
Primary Analysis: Walking Versus Inactive Controls
Overall, 45 studies compared walking with inactive controls in terms of reducing depressive symptoms. One study with adolescents was not included in the meta-analysis; it reported that walking significantly reduced depressive symptoms in female high school students with depression [43]. The studies included in each subgroup in the subgroup analysis are summarized in Table S5 in Multimedia Appendix 1 [46-51,60-67,71,73-76,78,80,81,83-85,87,88,90,91,93-95,97-104,106,108,110-112,114-116,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined,undefined].
The pooled results of the remaining 44 studies on adults indicated that walking could significantly reduce depressive symptoms (SMD −0.591, 95% CI −0.778 to −0.403; I2=84.8%; τ2=0.3008; P<.001; Table 2). In all the walking subgroups, except walking at a self-selected pace and group walking, walking could significantly reduce depressive symptoms when compared with the inactive controls (all P values were <.05). In the subgroup analysis, the intervention effects were significantly better in participants who were depressed (SMD −1.863, 95% CI −2.764 to −0.962) than in participants who were not depressed (SMD −0.442, 95% CI −0.604 to −0.280; P=.002), in interventions with a low dropout rate of 0% to 10% (SMD −0.779, 95% CI −1.086 to −0.471) than in those with a dropout rate of >10% (SMD −0.399, 95% CI −0.594 to −0.204; P=.04), in participants whose mean age was >60 years (SMD −0.885, 95% CI −1.261 to −0.509) than in those whose mean age was 30 to 60 years (SMD −0.417, 95% CI −0.659 to −0.176; P=.04), in interventions without motivation (SMD −0.763, 95% CI −1.045 to −0.480) than in those with motivation (SMD −0.292, 95% CI −0.476 to −0.107; P=.006), and in interventions with no pedometers (SMD −0.766, 95% CI −1.032 to −0.500) than in those with pedometers (SMD −0.292, 95% CI −0.532 to −0.052; P=.01). The forest plots are shown in Figure 3. In the multivariate meta-regression (R2=37.3%; Table S6 in Multimedia Appendix 1), baseline depression was significantly associated with a greater decline in depressive symptoms (SMD −1.158, 95% CI −1.851 to −0.466; P=.001).
Table 2. The effect of walking (all and different forms) on depressive symptoms compared with that of inactive controlsa in the main meta-analysis and subgroup analyses.
| Walking intervention, participant, and study characteristics | Studies, n | SMDb (95% CI) | I2, % | τ2 | P valuec | |
|---|---|---|---|---|---|---|
| All forms of walking | 44 | −0.591 (−0.778 to −0.403) | 84.8 | 0.3008 | —d | |
| Intervention duration (mo)e | .45 | |||||
| <3 | 15 | −0.743 (−1.199 to −0.287) | 83.7 | 0.6614 | ||
| 3‐6 | 26 | −0.545 (−0.778 to −0.323) | 86.5 | 0.2818 | ||
| Intensity | — | |||||
| At least moderate intensity | 22 | −0.485 (−0.719 to −0.252) | 82.2 | 0.2217 | ||
| Increasing intensity | 22 | −0.458 (−0.699 to −0.217) | 80.6 | 0.2238 | ||
| Frequency (number of times/wk)e | .29 | |||||
| <5 | 26 | −0.700 (−0.971 to −0.429) | 79.6 | 0.3674 | ||
| ≥5 | 12 | −0.472 (−0.791 to −0.153) | 85.9 | 0.2442 | ||
| Duration of each walk (min)e | .65 | |||||
| 10‐30 | 18 | −0.621 (−0.908 to −0.334) | 82.1 | 0.2701 | ||
| 35‐60 | 18 | −0.718 (−1.012 to −0.423) | 83.3 | 0.3072 | ||
| Walking pacee | .52 | |||||
| Self-selected pace | 7 | −0.415 (−1.037 to 0.206) | 91.6 | 0.6314 | ||
| Guided or set pace | 31 | −0.630 (−0.840 to −0.420) | 81 | 0.2462 | ||
| Walking formate | .95 | |||||
| Individual | 18 | −0.423 (−0.658 to −0.188) | 83.9 | 0.1892 | ||
| Group | 5 | −0.440 (−0.947 to 0.068) | 60.7 | 0.1964 | ||
| Walking locatione | .81 | |||||
| Outdoor | 8 | −0.765 (−1.198 to −0.333) | 79.2 | 0.2787 | ||
| Indoor | 14 | −0.839 (−1.268 to −0.410) | 81.4 | 0.5280 | ||
| Following instructions during walking | .06 | |||||
| Yes | 22 | −0.785 (−1.108 to −0.461) | 85.5 | 0.4825 | ||
| No | 22 | −0.411 (−0.634 to −0.189) | 81.9 | 0.1946 | ||
| Walking training | .90 | |||||
| Yes | 14 | −0.611 (−0.961 to −0.261) | 86.6 | 0.3545 | ||
| No | 30 | −0.583 (−0.815 to −0.351) | 84.2 | 0.3120 | ||
| Motivation (behavior change technique) f | .006 | |||||
| Yes | 16 | −0.292 (−0.476 to −0.107) | 70.1 | 0.0836 | ||
| No | 29 | −0.763 (−1.045 to −0.480) | 87.4 | 0.4774 | ||
| Pedometer | .01 | |||||
| Yes | 15 | −0.292 (−0.532 to −0.052) | 81.6 | 0.1547 | ||
| No | 29 | −0.766 (−1.032 to −0.500) | 83.1 | 0.4119 | ||
| Dropout rate (%) | .04 | |||||
| 0‐10 | 25 | −0.779 (−1.086 to −0.471) | 89.9 | 0.4968 | ||
| >10 | 17 | −0.399 (−0.594 to −0.204) | 59.7 | 0.0883 | ||
| Sample size, n | .42 | |||||
| <100 | 34 | −0.638 (−0.908 to −0.367) | 81.3 | 0.5126 | ||
| ≥100 | 10 | −0.479 (−0.753 to −0.204) | 89.9 | 0.1688 | ||
| Mean age (y)e | .04 | |||||
| 30‐60 | 26 | −0.417 (−0.659 to −0.176) | 82 | 0.2963 | ||
| >60 | 16 | −0.885 (−1.261 to −0.509) | 86.3 | 0.4682 | ||
| Baseline depressive symptoms | .002 | |||||
| Depressed | 5 | −1.863 (−2.764 to −0.962) | 86.4 | 0.8929 | ||
| Nondepressed | 39 | −0.442 (−0.604 to −0.280) | 77.5 | 0.1742 | ||
| Risk of bias | .75 | |||||
| Low or unclear | 34 | −0.610 (−0.848 to −0.371) | 82.4 | 0.3861 | ||
| High | 10 | −0.544 (−0.879 to −0.209) | 89 | 0.2271 | ||
Inactive controls included no intervention, waitlists, usual care, brief instructions on physical activity, and placebo meetings.
SMD: standardized mean difference (a higher SMD means more depressive symptoms).
Between-group difference.
Not applicable.
Studies with missing data on the specific subgroup were excluded from the corresponding subgroup analysis.
Two arms in one study can be categorized into two different subgroups.
Figure 3. Forest plot of the meta-analysis for the effect of walking on depressive symptoms compared with that of inactive controls [46-51,60-67,71,75,76,78,80,81,83-85,undefined,undefined,87,88,90,91,93-95,undefined,undefined,97-102,104,106,108,110-112,undefined,undefined,114,116]. DL: DerSimonian–Laird; SMD: standardized mean difference.
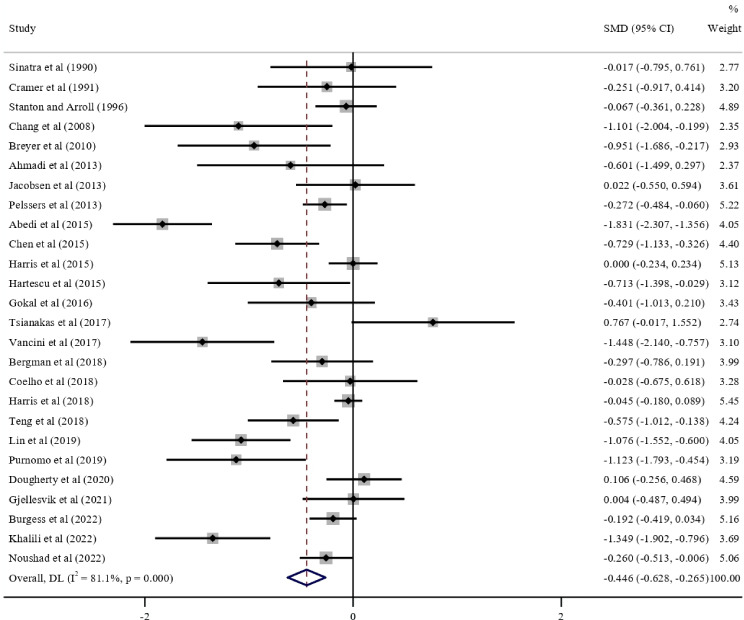
The pooled results of 26 studies on adults indicated that walking had significant positive effects on anxiety symptoms when compared with inactive controls (SMD −0.446, 95% CI −0.628 to −0.265; I2=81.1%; τ2=1.530; P<.001; Table 3). In all the walking subgroups, except walking at a self-selected pace and motivation, walking significantly reduced anxiety symptoms when compared with inactive controls (all P values were <.05). In the subgroup analysis, intervention effects were significantly better in interventions with a duration of <3 months (SMD −0.837, 95% CI −1.255 to −0.418) than in those with a duration of 3 to 6 months (SMD −0.312, 95% CI −0.538 to −0.085; P=.03), in interventions involving following instructions during walking (SMD −0.691, 95% CI −0.992 to −0.391) than in those not involving following instructions (SMD −0.273, 95% CI −0.488 to −0.057; P=.03), and in interventions without motivation (SMD −0.658, 95% CI −0.932 to −0.384) than in those with motivation (SMD −0.153, 95% CI −0.320 to 0.014; P=.002). The forest plots are shown in Figure 4. In the multivariate meta-regression (Table S6 in Multimedia Appendix 1), no factor was significantly associated with anxiety symptoms (R2=17%; all P values were >.05).
Table 3. The effect of walking (all and different forms) on anxiety symptoms compared with that of inactive controlsa in the main meta-analysis and subgroup analyses.
| Walking intervention, participant, and study characteristics | Studies, n | SMDb (95% CI) | I2, % | τ2 | P valuec | |
|---|---|---|---|---|---|---|
| All forms of walking | 26 | −0.446 (−0.628 to −0.265) | 81.1 | 0.1530 | —d | |
| Intervention duration (mo)e | .03 | |||||
| <3 | 8 | −0.837 (−1.255 to −0.418) | 80 | 0.2664 | ||
| 3‐6 | 16 | −0.312 (−0.538 to −0.085) | 81.2 | 0.1478 | ||
| Intensity | — | |||||
| At least moderate intensity | 14 | −0.317 (−0.523 to −0.111) | 70.8 | 0.0897 | ||
| Increasing intensity | 10 | −0.387 (−0.690 to −0.084) | 86.1 | 0.1727 | ||
| Frequency (number of times/wk)e | .55 | |||||
| <5 | 12 | −0.471 (−0.778 to −0.164) | 77.9 | 0.2048 | ||
| ≥5 | 11 | −0.355 (−0.576 to −0.135) | 67.4 | 0.0774 | ||
| Duration of each walk (min)e | .92 | |||||
| 10‐30 | 14 | −0.432 (−0.674 to −0.190) | 79 | 0.1360 | ||
| 35‐60 | 8 | −0.411 (−0.700 to −0.122) | 72.4 | 0.1151 | ||
| Walking pacee | .56 | |||||
| Self-selected pace | 4 | −0.625 (−1.459 to 0.208) | 93.5 | 0.6668 | ||
| Guided or set pace | 17 | −0.374 (−0.552 to −0.195) | 68.5 | 0.0765 | ||
| Walking formate | .41 | |||||
| Individual | 13 | −0.389 (−0.639 to −0.138) | 84.8 | 0.1590 | ||
| Group | 5 | −0.588 (−0.990 to −0.187) | 70.5 | 0.1347 | ||
| Walking locatione | .09 | |||||
| Outdoor | 5 | −0.991 (−1.567 to −0.414) | 82.5 | 0.3419 | ||
| Indoor | 5 | −0.416 (−0.733 to −0.099) | 32.2 | 0.0413 | ||
| Following instructions during walking | .03 | |||||
| Yes | 12 | −0.691 (−0.992 to −0.391) | 71.9 | 0.1872 | ||
| No | 14 | −0.273 (-0.488 to −0.057) | 82 | 0.1177 | ||
| Walking training | .67 | |||||
| Yes | 8 | −0.393 (−0.682 to −0.103) | 73.7 | 0.1143 | ||
| No | 18 | −0.475 (−0.714 to −0.236) | 83.9 | 0.1919 | ||
| Motivation (behavior change technique) f | .002 | |||||
| Yes | 11 | −0.153 (−0.320 to 0.014) | 57.1 | 0.0384 | ||
| No | 16 | −0.658 (−0.932 to −0.384) | 85.3 | 0.2296 | ||
| Pedometer | .17 | |||||
| Yes | 10 | −0.301 (−0.555 to −0.047) | 84.7 | 0.1228 | ||
| No | 16 | −0.554 (−0.810 to −0.298) | 74.1 | 0.1830 | ||
| Dropout rate (%)e | .42 | |||||
| 0‐10 | 11 | −0.542 (−0.860 to −0.224) | 87.2 | 0.2186 | ||
| >10 | 13 | −0.381 (−0.615 to −0.146) | 71.7 | 0.1151 | ||
| Sample size, n | .12 | |||||
| <100 | 16 | −0.576 (−0.853 to −0.299) | 67.9 | 0.2094 | ||
| ≥100 | 10 | −0.294 (−0.520 to −0.067) | 86 | 0.1068 | ||
| Mean age (y)e | .76 | |||||
| 30‐60 | 18 | −0.452 (−0.688 to −0.216) | 77.2 | 0.1801 | ||
| >60 | 7 | −0.523 (−0.929 to −0.118) | 86.5 | 0.2366 | ||
| Risk of bias | .72 | |||||
| Low or unclear | 19 | −0.423 (−0.620 to −0.226) | 65.1 | 0.1070 | ||
| High | 7 | −0.502 (−0.881 to −0.124) | 92 | 0.2268 | ||
Inactive controls included no intervention, waitlists, usual care, brief instructions on physical activity, and placebo meetings.
SMD: standardized mean difference (a higher SMD means more depressive symptoms).
Between-group difference.
Not applicable.
Studies with missing data on the specific subgroup were excluded from the corresponding subgroup analysis.
Two arms in one study can be categorized into two different subgroups.
Figure 4. Forest plot of the meta-analysis for the effect of walking on anxiety symptoms compared with that of inactive controls [46,47,50,60,64,71,73,74,78,80,83,84,87,90,91,93-95,undefined,undefined,99,101,103,106,111,114-116,undefined,undefined]. DL: DerSimonian–Laird; SMD: standardized mean difference.
The funnel plots are presented in Figure S1 in Multimedia Appendix 1 and are visually and statistically significantly asymmetrical (depressive symptoms: Egger test t=−2.02; P=.005; anxiety symptoms: Egger test t=−2.12; P=.01), which indicated the existence of small-study effects.
Secondary Analysis: Walking Versus Active Controls or Other Controls
The effects of walking on depressive and anxiety symptoms compared with those of active controls are summarized as forest plots in Figures S2 and S3 in Multimedia Appendix 1 [44,45,48,54,55,59,66,68,70-72,77,82,89,91,92,103,105,107,109,113,117,undefined,undefined]. There was no significant difference between walking and active controls in reducing depressive symptoms (RCTs: n=17; SMD −0.126, 95% CI −0.343 to 0.092; I2=58%; τ2=0.1058; P=.26) and anxiety symptoms (RCTs: n=14; SMD −0.053, 95% CI −0.311 to 0.206; I2=67.7%; τ2=0.1421; P=.69). The funnel plots are presented in Figure S4 in Multimedia Appendix 1. The Egger test showed no significant small-study effects for depressive symptoms (t=−1.02; P=.28) and anxiety symptoms (t=−0.60; P=.57).
The effects of walking on depressive symptoms were compared to those of light exercise, nutrition, and regular education in 6, 3, and 6 studies, respectively (Figure S5 in Multimedia Appendix 1 [44,46,52-54,56-58,69,79,86,96,98,105,undefined,undefined,undefined,undefined]). Walking had statistically significantly better effects on depressive symptoms than light exercise (SMD −0.426, 95% CI −0.766 to −0.086; I2=55.9%; τ2=0.1148; P=.046). There was no statistical difference when comparing walking with nutrition (SMD −0.091, 95% CI −0.541 to 0.358; I2=50.2%; τ2=0.0795; P=.69) and with regular education (SMD −0.208, 95% CI −0.476 to 0.060; I2=59.6%; τ2=0.0622; P=.13) in terms of depressive symptoms. There was no statistical difference between 4 studies that compared walking with light exercise in terms of anxiety symptoms (SMD −0.493, 95% CI −1.056 to 0.070; I2=65.1%; τ2=0.1611; P=.09).
Discussion
Principal Results
This systematic review of 75 studies assessed the effect of walking on depressive and anxiety symptoms and included comprehensive subgroup analyses, inactive controls, and active controls. The results indicated that walking could reduce depressive and anxiety symptoms in adults when compared with the inactive controls. The new findings in this review were that most of the walking subgroups had statistically significant beneficial effects, including different walking frequency, duration, location (indoor or outdoor), and format (group or individual) subgroups, and participants, regardless of whether they were depressed at baseline, benefited from the effects of walking. The effect of walking was also comparable to that of active controls.
Comparison With Prior Work
The results of the primary analysis, which showed that walking had an overall positive effect on remitted depressive and anxiety symptoms when compared with inactive controls, were consistent with previous meta-analyses on walking [22,23] and comparable to a meta-analysis of physical activity interventions [118]. However, our study did not find a dose-response relationship between walking, including walking frequency and the duration of each walk, and symptoms of depression or anxiety. In a recent meta-analysis of prospective cohort studies, the activity volume equivalents of 1.25 and 2.5 hours of brisk walking per week, which are shorter than the recommendation of 150 minutes of moderate-intensity physical activity per week from the World Health Organization (WHO) [119], were respectively associated with an 18% and 25% lower risk of depression [120]. These findings indicate that a shorter walking duration could still result in significant mental health benefits, albeit longer walks are better [121,122].
In most of the subgroup analyses, there was no statistical difference between the subgroups (all P values were >.05). This study found that both outdoor walking and indoor walking were effective in alleviating depression and anxiety, which is consistent with a previous meta-analysis on walking from 2012 [22]. It suggested that the effect of walking has no association with the location of walking (ie, indoors or outdoors). This might be important to homebound people (eg, older people or people who were home monitoring during the COVID-19 pandemic). Walking may also be effective regardless of whether people wear a pedometer and how old they are. In this review, walking at a self-selected pace was not effective on the selected mental health symptoms, whereas at least moderate-intensity walking and walking at a guided pace were effective. However, this study did not conduct a subgroup analysis of mild-intensity walking due to the limited number of relevant studies. These findings may indicate that walking at a guided or set pace (eg, a pace that can reach a certain speed or result in a certain heart rate), especially when such walking can reach a moderate intensity, is recommended, which is consistent with the WHO guideline that recommends moderate-intensity physical activity [119]. Future studies can closely examine the effects of mild-intensity walking on mental health.
In the remaining subgroup analyses, both adult participants who were depressed and those who were not depressed could benefit from walking, and participants who were depressed could benefit more. The meta-regression showed that baseline depression was the main factor that affected the effect of walking. The subgroup analysis of baseline anxiety was not conducted on anxiety symptoms due to a lack of data. Shorter intervention durations (<3 mo), walking without applying motivation, and walking without a pedometer were found to be significantly better than intervention durations of 3 to 6 months, walking while applying motivation, and walking with a pedometer, respectively. However, the latter three characteristics also had small and statistically significant positive effects on depressive or anxiety symptoms. The effects might have been confounded by other characteristics of walking interventions (eg, the intensity and frequency of walking), which may have contributed to the effectiveness of walking in the studies on the latter three characteristics. The lower adherence in these studies might also explain the smaller effects. A study found that adherence to physical exercise tends to decrease over time [123]. In addition, most of the walking interventions that applied motivation or involved a pedometer were unsupervised interventions that did not provide regular walking sessions. Supervised walking may be more likely to keep people walking regularly over longer time periods. Adherence to the prescribed dose of physical activity might be worse in unsupervised interventions, which rely on self-report measures to indicate adherence [124]. Better adherence to interventions was found to be associated with larger effects [125]. In this study, adherence was not analyzed in the subgroup analysis because the definition of adherence was different among studies. However, the larger effects in the studies with a low dropout rate of no more than 10% may support the beneficial effects of adherence to walking interventions.
In subgroup analyses, following or providing instructions during walking, such as in supervised walking or by including warm-up and cooldown components, was significantly better for anxiety symptoms than not following or providing instructions during walking. During this kind of walking, a certain walking pace might be settled. In addition, when following instructions, working memory has been shown to increase [126], and working memory training could reduce anxiety and depression vulnerability [127]. Hence, the settlement of walking pace and the involvement of working memory may serve as the moderators in the effect of walking instructions. Further studies are needed to confirm this mechanism.
The effect of walking was found to be comparable to that of active controls, which included other kinds of moderate-intensity physical exercise and other evidence-based interventions. This finding is consistent with previously reported results comparing the effects between physical exercise and other active controls. Exercise has been found to have a small but statistically nonsignificant effect when compared with psychological treatments or antidepressant medication [128]. However, there is no study comparing several different kinds of physical exercises (eg, via a network meta-analysis), including walking, in terms of their effects on mental health outcomes. Based on the existing evidence, walking (especially at a pace that can reach a certain speed, result in a certain heart rate, or reach moderate intensity) can be recommended as an alternative to other exercises and evidence-based interventions to improve mental health outcomes. Future studies can explore the dose-response relationship between walking and reduced mental health symptoms to provide more detailed instructions to people in need.
Based on the results found in this systematic review, incorporating walking into public health policies and initiatives can potentially decrease the prevalence of depression and enhance overall mental well-being. The results can be used to support mental health services in community and clinical settings that may incorporate walking as part of their prevention and treatment plans. Incorporating walking into indoor workplace wellness programs may improve employee mental health and productivity. Furthermore, health care care professionals should encourage and recommend at least moderate-intensity walking and such walking at a guided pace to the population to reduce depressive symptoms. Walking promotion should be included in the training and education for health care professionals and other health-related workers. They should recommend walking regularly to people in need, as well as supervise regular walking sessions, to ensure adherence to the walking exercise, and when resources are insufficient, priority should be given to people who are depressed.
Strengths and Limitations
The strengths of this systematic review and meta-analysis are that it included a large number of studies and confirmed the beneficial effects of walking on symptoms of depression and anxiety. A comprehensive subgroup analysis was conducted to explore the effects of different forms of walking. The effect of walking was also compared with that of different controls based on evidence, including inactive controls, active controls, and other controls.
This study has a few limitations that should be acknowledged. First, there was a high level of between-study heterogeneity in the primary analysis, although a subgroup analysis was performed to explore the potential reason for heterogeneity. Therefore, the results should be treated with caution when interpreting them and generalizing them to the real-world setting. Second, there existed small-study effects in the primary analysis, which could have been due to multiple reasons, such as clinical heterogeneity, outcome reporting bias, publication bias, and coincidence [129]. Third, most of the studies had an unclear (52/75, 69%) or high (17/75, 23%) risk of bias. This was due to the lack of reporting on allocation concealment and blinding of the outcome assessment. However, this is common among published RCTs. Previous literature suggested that only 6% of trials could be assessed as having an overall low risk of bias [130]. In this meta-analysis, a subgroup analysis was conducted to exclude the impact of studies with a high risk of bias and found that there was no significant difference between those with a high risk of bias and the remaining studies. Fourth, this study only measured the postintervention effect size. Further studies can be performed to measure the effects of the long-term maintenance of regular walking during follow-ups. In addition, as mentioned in the Comparison With Prior Work section, there is a lack of studies on mild-intensity walking. Future studies shall take a closer look at the effects of mild-intensity walking, besides the dose-response effects of walking on depression and anxiety. Fifth, most of the participants included in this meta-analysis did not have a diagnosis of depression or anxiety disorder. More walking interventions designed for diagnosed patients can be conducted in the future. Last, there is a lack of relevant trials on children and adolescents, which can be a future direction for research.
Conclusions
In summary, walking is an effective and promising intervention for reducing symptoms of depression and anxiety when compared with inactive controls. Its effects are comparable to active controls. Various forms of walking can serve as choices of treatment for people with symptoms of depression and anxiety. Integrating walking (especially at a pace that can reach moderate intensity) into public health initiatives can have potential benefits in reducing depression and anxiety. Future studies can explore the dose-response and long-term effects of walking on depression and anxiety.
Supplementary material
Abbreviations
- AMED
Allied and Complementary Medicine Database
- CENTRAL
Cochrane Central Register of Controlled Trials
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
randomized controlled trial
- SMD
standardized mean difference
- WHO
World Health Organization
Footnotes
Data Availability: The data sets generated and analyzed during this study are available from the corresponding author upon reasonable request.
Authors’ Contributions: ZX and DZ conceived the idea for the review, designed and performed the literature search, and coordinated the study. ZX, XZ, HD, and PMHC screened the records, extracted the data, and assessed the risk of bias. ZX coded the statistical analysis, figures, and appendix. ZX, DX, ZY, KWT, WZ, and DCCC interpreted the data. ZX and XZ wrote the first draft of the manuscript. SYSW supervised the study. All authors gave crucial feedback on the revised report and approved the final version of the manuscript.
Conflicts of Interest: None declared.
References
- 1.Depressive disorder (depression) World Health Organization. Mar 31, 2023. [20-05-2024]. https://www.who.int/news-room/fact-sheets/detail/depression URL. Accessed.
- 2.Anxiety disorders. World Health Organization. Sep 27, 2023. [20-05-2024]. https://www.who.int/news-room/fact-sheets/detail/anxiety-disorders URL. Accessed.
- 3.Depression and other common mental disorders: global health estimates. World Health Organization. 2017. [13-06-2024]. https://iris.who.int/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf URL. Accessed.
- 4.Lee B, Wang Y, Carlson SA, et al. National, state-level, and county-level prevalence estimates of adults aged ≥18 years self-reporting a lifetime diagnosis of depression - United States, 2020. MMWR Morb Mortal Wkly Rep. 2023 Jun 16;72(24):644–650. doi: 10.15585/mmwr.mm7224a1. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015 Sep;17(3):327–335. doi: 10.31887/DCNS.2015.17.3/bbandelow. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005 Jun;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 7.GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022 Feb;9(2):137–150. doi: 10.1016/S2215-0366(21)00395-3. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang R, Peng X, Song X, et al. The prevalence and risk of developing major depression among individuals with subthreshold depression in the general population. Psychol Med. 2023 Jun;53(8):3611–3620. doi: 10.1017/S0033291722000241. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosman RC, Have MT, de Graaf R, Muntingh AD, van Balkom AJ, Batelaan NM. Prevalence and course of subthreshold anxiety disorder in the general population: a three-year follow-up study. J Affect Disord. 2019 Mar 15;247:105–113. doi: 10.1016/j.jad.2019.01.018. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 10.VizHub - GBD Results. Institute for Health Metrics and Evaluation | Global Health Data Exchange. [01-05-2021]. http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b URL. Accessed.
- 11.Den Heijer AE, Groen Y, Tucha L, et al. Sweat it out? the effects of physical exercise on cognition and behavior in children and adults with ADHD: a systematic literature review. J Neural Transm (Vienna) 2017 Feb;124(Suppl 1):3–26. doi: 10.1007/s00702-016-1593-7. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paluch AE, Bajpai S, Bassett DR, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022 Mar;7(3):e219–e228. doi: 10.1016/S2468-2667(21)00302-9. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paluch AE, Gabriel KP, Fulton JE, et al. Steps per day and all-cause mortality in middle-aged adults in the Coronary Artery Risk Development in Young Adults study. JAMA Netw Open. 2021 Sep 1;4(9):e2124516. doi: 10.1001/jamanetworkopen.2021.24516. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly P, Williamson C, Niven AG, Hunter R, Mutrie N, Richards J. Walking on sunshine: scoping review of the evidence for walking and mental health. Br J Sports Med. 2018 Jun;52(12):800–806. doi: 10.1136/bjsports-2017-098827. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 15.Murtagh EM, Nichols L, Mohammed MA, Holder R, Nevill AM, Murphy MH. The effect of walking on risk factors for cardiovascular disease: an updated systematic review and meta-analysis of randomised control trials. Prev Med. 2015 Mar;72:34–43. doi: 10.1016/j.ypmed.2014.12.041. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MH, Nevill AM, Murtagh EM, Holder RL. The effect of walking on fitness, fatness and resting blood pressure: a meta-analysis of randomised, controlled trials. Prev Med. 2007 May;44(5):377–385. doi: 10.1016/j.ypmed.2006.12.008. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 17.Ballard AM, Davis A, Wong B, Lyn R, Thompson WR. The effects of exclusive walking on lipids and lipoproteins in women with overweight and obesity: a systematic review and meta-analysis. Am J Health Promot. 2022 Feb;36(2):328–339. doi: 10.1177/08901171211048135. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor SR, Tully MA, Ryan B, et al. Walking exercise for chronic musculoskeletal pain: systematic review and meta-analysis. Arch Phys Med Rehabil. 2015 Apr;96(4):724–734.e3. doi: 10.1016/j.apmr.2014.12.003. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 19.Richardson CR, Newton TL, Abraham JJ, Sen A, Jimbo M, Swartz AM. A meta-analysis of pedometer-based walking interventions and weight loss. Ann Fam Med. 2008;6(1):69–77. doi: 10.1370/afm.761. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu S, Cai X, Schumann U, Velders M, Sun Z, Steinacker JM. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta-analysis. PLoS One. 2014 Oct 17;9(10):e109767. doi: 10.1371/journal.pone.0109767. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly P, Kahlmeier S, Götschi T, et al. Systematic review and meta-analysis of reduction in all-cause mortality from walking and cycling and shape of dose response relationship. Int J Behav Nutr Phys Act. 2014 Oct 24;11:132. doi: 10.1186/s12966-014-0132-x. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson R, Robertson A, Jepson R, Maxwell M. Walking for depression or depressive symptoms: a systematic review and meta-analysis. Ment Health Phys Act. 2012 Jun;5(1):66–75. doi: 10.1016/j.mhpa.2012.03.002. doi. [DOI] [Google Scholar]
- 23.Hanson S, Jones A. Is there evidence that walking groups have health benefits? a systematic review and meta-analysis. Br J Sports Med. 2015 Jun;49(11):710–715. doi: 10.1136/bjsports-2014-094157. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pentland V, Spilsbury S, Biswas A, Mottola MF, Paplinskie S, Mitchell MS. Does walking reduce postpartum depressive symptoms? a systematic review and meta-analysis of randomized controlled trials. J Womens Health (Larchmt) 2022 Apr;31(4):555–563. doi: 10.1089/jwh.2021.0296. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 25.Grassini S. A systematic review and meta-analysis of nature walk as an intervention for anxiety and depression. J Clin Med. 2022 Mar 21;11(6):1731. doi: 10.3390/jcm11061731. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Health care. US Centers for Disease Control and Prevention. [13-06-2024]. https://www.cdc.gov/physicalactivity/activepeoplehealthynation/everyone-can-be-involved/health-care.html URL. Accessed.
- 27.Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011 Jan;22(1):128; author reply 128. doi: 10.1097/EDE.0b013e3181fe7825. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Cochrane; 2019. [13-06-2024]. https://training.cochrane.org/handbook URL. Accessed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner M, Boshart ML, Yeguez CE, Desai KM, Sandberg DE. Coming up short: risks of bias in assessing psychological outcomes in growth hormone therapy for short stature. J Clin Endocrinol Metab. 2016 Jan;101(1):23–30. doi: 10.1210/jc.2015-3256. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 30.Mohr DC, Ho J, Hart TL, et al. Control condition design and implementation features in controlled trials: a meta-analysis of trials evaluating psychotherapy for depression. Transl Behav Med. 2014 Dec;4(4):407–423. doi: 10.1007/s13142-014-0262-3. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold SM, Enck P, Hasselmann H, et al. Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. Lancet Psychiatry. 2017 Sep;4(9):725–732. doi: 10.1016/S2215-0366(17)30153-0. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 32.Brinsley J, Schuch F, Lederman O, et al. Effects of yoga on depressive symptoms in people with mental disorders: a systematic review and meta-analysis. Br J Sports Med. 2021 Sep;55(17):992–1000. doi: 10.1136/bjsports-2019-101242. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Lee EKO, Wu T, et al. The effects of tai chi on depression, anxiety, and psychological well-being: a systematic review and meta-analysis. Int J Behav Med. 2014 Aug;21(4):605–617. doi: 10.1007/s12529-013-9351-9. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 34.Reangsing C, Rittiwong T, Schneider JK. Effects of mindfulness meditation interventions on depression in older adults: a meta-analysis. Aging Ment Health. 2021 Jul;25(7):1181–1190. doi: 10.1080/13607863.2020.1793901. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 35.Feng CY, Chu H, Chen CH, et al. The effect of cognitive behavioral group therapy for depression: a meta-analysis 2000-2010. Worldviews Evid Based Nurs. 2012 Feb;9(1):2–17. doi: 10.1111/j.1741-6787.2011.00229.x. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 36.Li SYH, Bressington D. The effects of mindfulness-based stress reduction on depression, anxiety, and stress in older adults: a systematic review and meta-analysis. Int J Ment Health Nurs. 2019 Jun;28(3):635–656. doi: 10.1111/inm.12568. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Cheng P, Wu Y, et al. The effects of art therapy on anxiety and depression in breast cancer patients: an updated meta-analysis. Eur J Cancer Care (Engl) 2020 Sep;29(5):e13266. doi: 10.1111/ecc.13266. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 38.Watt JA, Goodarzi Z, Veroniki AA, et al. Comparative efficacy of interventions for reducing symptoms of depression in people with dementia: systematic review and network meta-analysis. BMJ. 2021 Mar 24;372:n532. doi: 10.1136/bmj.n532. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010 Apr;1(2):97–111. doi: 10.1002/jrsm.12. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 40.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012 Jun;41(3):818–827. doi: 10.1093/ije/dys041. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001 Oct;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 43.Roshan VD, Pourasghar M, Mohammadian Z. The efficacy of intermittent walking in water on the rate of MHPG sulfate and the severity of depression. Iran J Psychiatry Behav Sci. 2011;5(2):26–31. Medline. [PMC free article] [PubMed] [Google Scholar]
- 44.Minor MA, Hewett JE, Webel RR, Anderson SK, Kay DR. Efficacy of physical conditioning exercise in patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1989 Nov;32(11):1396–1405. doi: 10.1002/anr.1780321108. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 45.Sexton H, Maere A, Dahl NH. Exercise intensity and reduction in neurotic symptoms: a controlled follow‐up study. Acta Psychiatr Scand. 1989 Sep;80(3):231–235. doi: 10.1111/j.1600-0447.1989.tb01332.x. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 46.Sinatra ST, Allen GJ, Camaione DN, Abraham A. Effects of continuous passive motion, walking, and a placebo intervention on physical and psychological well-being. [14-06-2024];J Cardiopulm Rehabil. 1990 Aug;10(8):279–286. https://heartmdinstitute.com/wp-content/uploads/2013/04/EffectsContinPassiveMotion.pdf URL. Accessed. [Google Scholar]
- 47.Cramer SR, Nieman DC, Lee JW. The effects of moderate exercise training on psychological well-being and mood state in women. J Psychosom Res. 1991;35(4-5):437–449. doi: 10.1016/0022-3999(91)90039-q. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 48.McNeil JK, LeBlanc EM, Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991 Sep;6(3):487–488. doi: 10.1037//0882-7974.6.3.487. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 49.Palmer LK. Effects of a walking program on attributional style, depression, and self-esteem in women. Percept Mot Skills. 1995 Dec;81(3 Pt 1):891–898. doi: 10.2466/pms.1995.81.3.891. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 50.Stanton JM, Arroll B. The effect of moderate exercise on mood in mildly hypertensive volunteers: a randomized controlled trial. J Psychosom Res. 1996 Jun;40(6):637–642. doi: 10.1016/0022-3999(95)00643-5. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 51.Moreau KL. The University of Tennessee; 1999. [14-06-2024]. The effects of walking volume on blood pressure in hypertensive postmenopausal women [Dissertation]https://trace.tennessee.edu/cgi/viewcontent.cgi?article=10494&context=utk_graddiss URL. Accessed. [Google Scholar]
- 52.Nieman DC, Custer WF, Butterworth DE, Utter AC, Henson DA. Psychological response to exercise training and/or energy restriction in obese women. J Psychosom Res. 2000 Jan;48(1):23–29. doi: 10.1016/s0022-3999(99)00066-5. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 53.King AC, Baumann K, O’Sullivan P, Wilcox S, Castro C. Effects of moderate-intensity exercise on physiological, behavioral, and emotional responses to family caregiving: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2002 Jan;57(1):M26–M36. doi: 10.1093/gerona/57.1.m26. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 54.Penninx BWJH, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci. 2002 Mar;57(2):P124–P132. doi: 10.1093/geronb/57.2.p124. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong K, Edwards H. The effectiveness of a pram‐walking exercise programme in reducing depressive symptomatology for postnatal women. Int J Nurs Pract. 2004 Aug;10(4):177–194. doi: 10.1111/j.1440-172X.2004.00478.x. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 56.Motl RW, Konopack JF, McAuley E, Elavsky S, Jerome GJ, Marquez DX. Depressive symptoms among older adults: long-term reduction after a physical activity intervention. J Behav Med. 2005 Aug;28(4):385–394. doi: 10.1007/s10865-005-9005-5. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 57.Gary R. Exercise self-efficacy in older women with diastolic heart failure: results of a walking program and education intervention. J Gerontol Nurs. 2006 Jul;32(7):31–39. doi: 10.3928/00989134-20060701-05. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 58.Knubben K, Reischies FM, Adli M, Schlattmann P, Bauer M, Dimeo F. A randomised, controlled study on the effects of a short-term endurance training programme in patients with major depression. Br J Sports Med. 2007 Jan;41(1):29–33. doi: 10.1136/bjsm.2006.030130. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bircan C, Karasel SA, Akgün B, El O, Alper S. Effects of muscle strengthening versus aerobic exercise program in fibromyalgia. Rheumatol Int. 2008 Apr;28(6):527–532. doi: 10.1007/s00296-007-0484-5. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 60.Chang PH, Lai YH, Shun SC, et al. Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manage. 2008 May;35(5):524–534. doi: 10.1016/j.jpainsymman.2007.06.013. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 61.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008 Jul;35(4):635–642. doi: 10.1188/08.ONF.635-642. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 62.Smith PS, Thompson M. Treadmill training post stroke: are there any secondary benefits? a pilot study. Clin Rehabil. 2008;22(10-11):997–1002. doi: 10.1177/0269215508088988. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 63.Robichaud KP. Middle Tennessee State University; 2008. [14-06-2024]. The effects of an exercise intervention on the psychological well-being of postpartum women [Dissertation]https://jewlscholar.mtsu.edu/server/api/core/bitstreams/9497ccbb-dc6a-4c7e-bf34-d32929f93f12/content URL. Accessed. [Google Scholar]
- 64.Breyer MK, Breyer-Kohansal R, Funk GC, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res. 2010 Aug 22;11(1):1–9. doi: 10.1186/1465-9921-11-112. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chao PJ. University of Hawaii at Manoa; 2010. [14-06-2024]. A group randomized trial to examine the feasibility and effects of pedometer use and self-monitoring of daily walking in people with severe and persistent mental illnesses [Thesis]https://scholarspace.manoa.hawaii.edu/items/872e7c70-4ed6-4064-af15-f36bec83d5b7 URL. Accessed. [Google Scholar]
- 66.Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res. 2010 Aug;69(2):119–131. doi: 10.1016/j.jpsychores.2010.01.013. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins TC, Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011 Oct;34(10):2174–2179. doi: 10.2337/dc10-2399. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCaffrey R, Liehr P, Gregersen T, Nishioka R. Garden walking and art therapy for depression in older adults: a pilot study. Res Gerontol Nurs. 2011 Oct;4(4):237–242. doi: 10.3928/19404921-20110201-01. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 69.Maki Y, Ura C, Yamaguchi T, et al. Effects of intervention using a community‐based walking program for prevention of mental decline: a randomized controlled trial. J Am Geriatr Soc. 2012 Mar;60(3):505–510. doi: 10.1111/j.1532-5415.2011.03838.x. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 70.Ridsdale L, Hurley M, King M, McCrone P, Donaldson N. The effect of counselling, graded exercise and usual care for people with chronic fatigue in primary care: a randomized trial. Psychol Med. 2012 Oct;42(10):2217–2224. doi: 10.1017/S0033291712000256. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmadi A, Arastoo AA, Nikbakht M, Zahednejad S, Rajabpour M. Comparison of the effect of 8 weeks aerobic and yoga training on ambulatory function, fatigue and mood status in MS patients. Iran Red Crescent Med J. 2013 Jun;15(6):449–454. doi: 10.5812/ircmj.3597. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Faulkner J, Westrupp N, Rousseau J, Lark S. A randomized controlled trial to assess the effect of self-paced walking on task-specific anxiety in cardiac rehabilitation patients. J Cardiopulm Rehabil Prev. 2013;33(5):292–296. doi: 10.1097/HCR.0b013e3182a0295c. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 73.Jacobsen PB, Phillips KM, Jim HSL, et al. Effects of self‐directed stress management training and home‐based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psychooncology. 2013 Jun;22(6):1229–1235. doi: 10.1002/pon.3122. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 74.Pelssers J, Delecluse C, Opdenacker J, Kennis E, Van Roie E, Boen F. "Every step counts!": effects of a structured walking intervention in a community-based senior organization. J Aging Phys Act. 2013 Apr;21(2):167–185. doi: 10.1123/japa.21.2.167. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 75.Bernard P, Ninot G, Bernard PL, et al. Effects of a six-month walking intervention on depression in inactive post-menopausal women: a randomized controlled trial. Aging Ment Health. 2015;19(6):485–492. doi: 10.1080/13607863.2014.948806. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 76.Prakhinkit S, Suppapitiporn S, Tanaka H, Suksom D. Effects of Buddhism walking meditation on depression, functional fitness, and endothelium-dependent vasodilation in depressed elderly. J Altern Complement Med. 2014 May;20(5):411–416. doi: 10.1089/acm.2013.0205. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 77.Van Hoecke AS, Delecluse C, Bogaerts A, Boen F. Effects of need-supportive physical activity counseling on well-being: a 2-year follow-up among sedentary older adults. J Phys Act Health. 2014 Nov;11(8):1492–1502. doi: 10.1123/jpah.2012-0497. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 78.Abedi P, Nikkhah P, Najar S. Effect of pedometer-based walking on depression, anxiety and insomnia among postmenopausal women. Climacteric. 2015;18(6):841–845. doi: 10.3109/13697137.2015.1065246. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 79.Bellon K, Kolakowsky-Hayner S, Wright J, et al. A home-based walking study to ameliorate perceived stress and depressive symptoms in people with a traumatic brain injury. Brain Inj. 2015;29(3):313–319. doi: 10.3109/02699052.2014.974670. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 80.Chen HM, Tsai CM, Wu YC, Lin KC, Lin CC. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer. 2015 Feb 3;112(3):438–445. doi: 10.1038/bjc.2014.612. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cugusi L, Solla P, Serpe R, et al. Effects of a Nordic walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson’s disease. NeuroRehabilitation. 2015;37(2):245–254. doi: 10.3233/NRE-151257. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 82.Ferreira L, Tanaka K, Santos-Galduróz RF, Galduróz JCF. Respiratory training as strategy to prevent cognitive decline in aging: a randomized controlled trial. Clin Interv Aging. 2015 Mar 20;10:593–603. doi: 10.2147/CIA.S79560. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harris T, Kerry SM, Victor CR, et al. A primary care nurse-delivered walking intervention in older adults: PACE (pedometer accelerometer consultation evaluation)-Lift cluster randomised controlled trial. PLoS Med. 2015 Feb 17;12(2):e1001783. doi: 10.1371/journal.pmed.1001783. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hartescu I, Morgan K, Stevinson CD. Increased physical activity improves sleep and mood outcomes in inactive people with insomnia: a randomized controlled trial. J Sleep Res. 2015 Oct;24(5):526–534. doi: 10.1111/jsr.12297. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 85.Abd El-Kader SM, Al-Jiffri OH. Aerobic exercise improves quality of life, psychological well-being and systemic inflammation in subjects with Alzheimer’s disease. Afr Health Sci. 2016 Dec;16(4):1045–1055. doi: 10.4314/ahs.v16i4.22. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abdelhamid ZSA, Serry ZM, Elnahas NMG, Ammar NM. Serum serotonin response to aerobic exercise verus phoenix. [14-06-2024];International Journal of PharmTech Research. 2016 9(10):108–114. https://sphinxsai.com/2016/ph_vol9_no10/1/(108-114)V9N10PT.pdf URL. Accessed. [Google Scholar]
- 87.Gokal K, Wallis D, Ahmed S, Boiangiu I, Kancherla K, Munir F. Effects of a self-managed home-based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: a randomised controlled trial. Support Care Cancer. 2016 Mar;24(3):1139–1166. doi: 10.1007/s00520-015-2884-5. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 88.Picelli A, Varalta V, Melotti C, et al. Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson’s disease: a pilot, single-blind, randomized controlled trial. Funct Neurol. 2016;31(1):25–31. doi: 10.11138/fneur/2016.31.1.025. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shahabi L, Naliboff BD, Shapiro D. Self-regulation evaluation of therapeutic yoga and walking for patients with irritable bowel syndrome: a pilot study. Psychol Health Med. 2016;21(2):176–188. doi: 10.1080/13548506.2015.1051557. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 90.Tsianakas V, Harris J, Ream E, et al. CanWalk: a feasibility study with embedded randomised controlled trial pilot of a walking intervention for people with recurrent or metastatic cancer. BMJ Open. 2017 Feb 15;7(2):e013719. doi: 10.1136/bmjopen-2016-013719. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vancini RL, Rayes ABR, de Lira CAB, Sarro KJ, Andrade MS. Pilates and aerobic training improve levels of depression, anxiety and quality of life in overweight and obese individuals. Arq Neuropsiquiatr. 2017 Dec;75(12):850–857. doi: 10.1590/0004-282X20170149. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 92.Van Schaardenburgh M, Wohlwend M, Rognmo Ø, Mattsson E. Calf raise exercise increases walking performance in patients with intermittent claudication. J Vasc Surg. 2017 May;65(5):1473–1482. doi: 10.1016/j.jvs.2016.12.106. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 93.Bergman F, Wahlström V, Stomby A, et al. Treadmill workstations in office workers who are overweight or obese: a randomised controlled trial. Lancet Public Health. 2018 Nov;3(11):e523–e535. doi: 10.1016/S2468-2667(18)30163-4. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 94.Coelho CM, Reboredo MM, Valle FM, et al. Effects of an unsupervised pedometer-based physical activity program on daily steps of adults with moderate to severe asthma: a randomized controlled trial. J Sports Sci. 2018 May;36(10):1186–1193. doi: 10.1080/02640414.2017.1364402. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 95.Harris T, Kerry S, Victor C, et al. A pedometer-based walking intervention in 45- to 75-year-olds, with and without practice nurse support: the PACE-UP three-arm cluster RCT. Health Technol Assess. 2018 Jun;22(37):1–274. doi: 10.3310/hta22370. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katz P, Margaretten M, Gregorich S, Trupin L. Physical activity to reduce fatigue in rheumatoid arthritis: a randomized controlled trial. Arthritis Care Res (Hoboken) 2018 Jan;70(1):1–10. doi: 10.1002/acr.23230. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 97.Kuo MC, Chen CM, Jeng C. A randomized controlled trial of the prescribed stepper walking program in preventing frailty among the dwelling elderly: application of comprehensive geriatric assessment. Top Geriatr Rehabil. 2018 Jul;34(3):223–333. doi: 10.1097/TGR.0000000000000198. doi. [DOI] [Google Scholar]
- 98.Putra ES, Wasita B, Anantanyu S. A randomised trial on walking exercise and banana consumption on self-reported depression symptoms among female adolescents in Surakarta, Indonesia. [14-06-2024];Mal J Nutr. 2018 24(3):467–473. https://nutriweb.org.my/mjn/publication/24-3/o.pdf URL. Accessed. [Google Scholar]
- 99.Teng HC, Yeh ML, Wang MH. Walking with controlled breathing improves exercise tolerance, anxiety, and quality of life in heart failure patients: a randomized controlled trial. Eur J Cardiovasc Nurs. 2018 Dec;17(8):717–727. doi: 10.1177/1474515118778453. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 100.Abdelbasset WK, Alqahtani BA. A randomized controlled trial on the impact of moderate-intensity continuous aerobic exercise on the depression status of middle-aged patients with congestive heart failure. Medicine (Baltimore) 2019 Apr;98(17):e15344. doi: 10.1097/MD.0000000000015344. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lin FL, Yeh ML, Lai YH, Lin KC, Yu CJ, Chang JS. Two‐month breathing‐based walking improves anxiety, depression, dyspnoea and quality of life in chronic obstructive pulmonary disease: a randomised controlled study. J Clin Nurs. 2019 Oct;28(19-20):3632–3640. doi: 10.1111/jocn.14960. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 102.Miyamoto ST, Valim V, Carletti L, et al. Supervised walking improves cardiorespiratory fitness, exercise tolerance, and fatigue in women with primary Sjögren’s syndrome: a randomized-controlled trial. Rheumatol Int. 2019 Feb;39(2):227–238. doi: 10.1007/s00296-018-4213-z. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 103.Purnomo KI, Doewes M, Suroto S, Murti B, Giri MKW. The combination effect of brisk walking and relaxation toward hs-crp and anxiety levels in subject with central obesity in Singaraja, Bali. Bali Medical Journal. 2019;8(1):294–298. doi: 10.15562/bmj.v8i1.1287. doi. [DOI] [Google Scholar]
- 104.Shi L, Welsh RS, Lopes S, et al. A pilot study of mindful walking training on physical activity and health outcomes among adults with inadequate activity. Complement Ther Med. 2019 Jun;44:116–122. doi: 10.1016/j.ctim.2019.03.009. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 105.Suh JH, Kim H, Jung GP, Ko JY, Ryu JS. The effect of lumbar stabilization and walking exercises on chronic low back pain: a randomized controlled trial. Medicine (Baltimore) 2019 Jun;98(26):e16173. doi: 10.1097/MD.0000000000016173. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dougherty CM, Burr RL, Kudenchuk PJ, Glenny RW. Aerobic exercise effects on quality of life and psychological distress after an implantable cardioverter defibrillator. J Cardiopulm Rehabil Prev. 2020 Mar;40(2):94–101. doi: 10.1097/HCR.0000000000000444. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rezola-Pardo C, Rodriguez-Larrad A, Gomez-Diaz J, et al. Comparison between multicomponent exercise and walking interventions in long-term nursing homes: a randomized controlled trial. Gerontologist. 2020 Sep 15;60(7):1364–1373. doi: 10.1093/geront/gnz177. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 108.Sheshadri A, Kittiskulnam P, Lazar AA, Johansen KL. A walking intervention to increase weekly steps in dialysis patients: a pilot randomized controlled trial. Am J Kidney Dis. 2020 Apr;75(4):488–496. doi: 10.1053/j.ajkd.2019.07.026. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yentür SB, Ataş N, Öztürk MA, Oskay D. Comparison of the effectiveness of pilates exercises, aerobic exercises, and pilates with aerobic exercises in patients with rheumatoid arthritis. Ir J Med Sci. 2021 Aug;190(3):1027–1034. doi: 10.1007/s11845-020-02412-2. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 110.Bade BC, Gan G, Li F, et al. Randomized trial of physical activity on quality of life and lung cancer biomarkers in patients with advanced stage lung cancer: a pilot study. BMC Cancer. 2021 Apr 1;21(1):352. doi: 10.1186/s12885-021-08084-0. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gjellesvik TI, Becker F, Tjønna AE, et al. Effects of high-intensity interval training after stroke (the HIIT Stroke Study) on physical and cognitive function: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2021 Sep;102(9):1683–1691. doi: 10.1016/j.apmr.2021.05.008. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 112.Hammer MJ, Eckardt P, Cartwright F, Miaskowski C. Prescribed walking for glycemic control and symptom management in patients without diabetes undergoing chemotherapy. Nurs Res. 2021;70(1):6–14. doi: 10.1097/NNR.0000000000000468. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 113.Saavedra JM, Kristjánsdóttir H, Gunnarsson SB, García-Hermoso A. Effects of 2 physical exercise programs (circuit training and brisk walk) carried out during working hours on multidimensional components of workers' health: a pilot study. Int J Occup Med Environ Health. 2021 Jan 7;34(1):39–51. doi: 10.13075/ijomeh.1896.01647. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 114.Burgess DJ, Campbell EH, Hammett P, et al. Taking ACTION to reduce pain: a randomized clinical trial of a walking-focused, proactive coaching intervention for Black patients with chronic musculoskeletal pain. J Gen Intern Med. 2022 Nov;37(14):3585–3593. doi: 10.1007/s11606-021-07376-2. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khalili S, Shirinkam F, Ghadimi R, Karimi H. The effect of group walking program on social physique anxiety and the risk of eating disorders in aged women: a randomized clinical trial study. Appl Nurs Res. 2022 Apr;64:151555. doi: 10.1016/j.apnr.2021.151555. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 116.Noushad S, Ansari B, Ahmed S. Effect of nature-based physical activity on post-traumatic growth among healthcare providers with post-traumatic stress. Stress Health. 2022 Oct;38(4):813–826. doi: 10.1002/smi.3135. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 117.Reed JL, Terada T, Cotie LM, et al. The effects of high-intensity interval training, Nordic walking and moderate-to-vigorous intensity continuous training on functional capacity, depression and quality of life in patients with coronary artery disease enrolled in cardiac rehabilitation: a randomized controlled trial (CRX study) Prog Cardiovasc Dis. 2022;70:73–83. doi: 10.1016/j.pcad.2021.07.002. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 118.Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;9(3):366–378. doi: 10.1080/17437199.2015.1022901. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 119.World Health Organization . Guidelines on Physical Activity and Sedentary Behaviour. World Health Organization; 2020. [PubMed] [Google Scholar]
- 120.Pearce M, Garcia L, Abbas A, et al. Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiatry. 2022 Jun 1;79(6):550–559. doi: 10.1001/jamapsychiatry.2022.0609. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miller JC, Krizan Z. Walking facilitates positive affect (even when expecting the opposite) Emotion. 2016 Aug;16(5):775–785. doi: 10.1037/a0040270. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 122.Zhu Z, Chen H, Ma J, He Y, Chen J, Sun J. Exploring the relationship between walking and emotional health in China. Int J Environ Res Public Health. 2020 Nov 27;17(23):8804. doi: 10.3390/ijerph17238804. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lopes S, Félix G, Mesquita-Bastos J, Figueiredo D, Oliveira J, Ribeiro F. Determinants of exercise adherence and maintenance among patients with hypertension: a narrative review. Rev Cardiovasc Med. 2021 Dec 22;22(4):1271–1278. doi: 10.31083/j.rcm2204134. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 124.Creasy SA, Rogers RJ, Davis KK, Gibbs BB, Kershaw EE, Jakicic JM. Effects of supervised and unsupervised physical activity programmes for weight loss. Obes Sci Pract. 2017 May 5;3(2):143–152. doi: 10.1002/osp4.107. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fuhr K, Schröder J, Berger T, et al. The association between adherence and outcome in an internet intervention for depression. J Affect Disord. 2018 Mar 15;229:443–449. doi: 10.1016/j.jad.2017.12.028. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 126.Dunham S, Lee E, Persky AM. The psychology of following instructions and its implications. Am J Pharm Educ. 2020 Aug;84(8):ajpe7779. doi: 10.5688/ajpe7779. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Beloe P, Derakshan N. Adaptive working memory training can reduce anxiety and depression vulnerability in adolescents. Dev Sci. 2020 Jul;23(4):e12831. doi: 10.1111/desc.12831. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 128.Kvam S, Kleppe CL, Nordhus IH, Hovland A. Exercise as a treatment for depression: a meta-analysis. J Affect Disord. 2016 Sep 15;202:67–86. doi: 10.1016/j.jad.2016.03.063. doi. Medline. [DOI] [PubMed] [Google Scholar]
- 129.Schwarzer G, Carpenter JR, Rücke G. Meta-Analysis With R. Springer Cham; 2015. Small-study effects in meta-analysis; pp. 107–141. doi. [DOI] [Google Scholar]
- 130.Jørgensen L, Paludan-Müller AS, Laursen DRT, et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. 2016 May 10;5:80. doi: 10.1186/s13643-016-0259-8. doi. Medline. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.